Introduction
Cardiovascular ischaemic diseases are a major contributor to death and disability worldwide [1]. Myocardial ischaemic loss in acute myocardial infarction remains the most significant contributor to chronic heart failure and sudden cardiac death as a single (usually large) myocardial infarct or multi-infarct ischaemic injury [1]. Despite the progress in pharmacological and invasive management of acute myocardial infarction, the prevalence of ischaemic heart failure will grow substantially in the next decades [2]. Novel therapeutic approaches, including stimulation of cardiac repair and regeneration, are needed [2, 3]. Observer-independent, reproducible imaging techniques play a fundamental role in objective evaluation of both conventional (such as surgical or percutaneous) myocardial revascularization and novel therapeutic approaches to reduce myocardial ischaemia and improve contractility. To be applicable to clinical practice, the clinical study design and data should best be rooted in everyday clinical practice. Current techniques used both in clinical studies and in everyday clinical practice include 2- and 3-dimensional echocardiography, cardiac magnetic resonance imaging (cMRI), single-photon emission computed tomography (SPECT) and positron emission tomography (PET); each of these has its strengths and limitations.
We review present evidence on the role of single-photon emission computed tomography as a technique that may offer, through being observer-independent, the most objective evaluation of evolution of left ventricular perfusion, volumes and ejection fraction.
SPECT amongst other imaging methods
Accurate and reproducible assessment of left ventricular (LV) ejection fraction (LVEF) is one of the most important objectives of cardiac imaging. LVEF refers to the fraction of LV end-diastolic volume ejected during systole. The impact of preserved or reduced LVEF may answer fundamental questions regarding the patient’s prognosis, especially in myocardial infarction and heart failure. Several different imaging modalities have been thus far used for more or less accurate evaluation of LV volumes and LVEF. Transthoracic echocardiography is probably the most widely used cardiac imaging modality in routine clinical practice. However, echocardiography has some fundamental limitations, including challenges in exact measurement of LV volume due to the complex LV cavity geometry, manual delineation and observer dependence. Thus it may offer limited research value to detect modest improvements in global LV contractility, although technical improvements (such as contrast echocardiography [4–6] or speckle-tracking imaging [7, 8]) tend to offer more accurate estimations. Still, echocardiographic assessment of LV function is challenging even with the more recent and more automated methods. LVEF is also frequently determined using cMRI or gated SPECT (GSPECT) imaging. Usefulness of the three modalities has been recently confirmed by a large, multi-centre study, where 2032 patients with coronary artery disease and LVEF of 35% or less were compared. In this study correlation of LVEF between modalities ranged from r = 0.493 (for biplane echocardiography and cardiovascular magnetic resonance) to r = 0.660 (for cardiovascular magnetic resonance and gated single-photon emission computed tomography). There was no systematic overestimation or underestimation of LVEF for any modality [9]. These methods are nowadays used in daily routine practice for clinical decision making. However, there are differences in their precision that may play a particularly important role in detecting subtle changes in myocardial perfusion and contractility.
Development of G-SPECT imaging
The history of nuclear cardiology began in 1957, when Hal Anger developed the very first scintillation gamma camera, which among other uses was able to provide images of the distribution of radioactivity in the patient’s heart [10]. With the further technological advances in the 1970s, physicians acquired a tool to non-invasively measure ventricular function, initially with end-systolic and end-diastolic equilibrium radionuclide ventriculography with the help of an ECG-gating device. In the following years the first medical imaging computers were introduced, which led to the development of the multiple gated acquisition (MUGA) scan, thus allowing nuclear cardiology to become a useful, routine research and clinical tool. However, the G-SPECT technique was not widely used until the 1990s, when 99mTc-MIBI became approved for use in both the US and Europe. 99mTc-MIBI offered much greater myocardial uptake and improved image count over previously used techniques, mainly 201Tl, offering the possibility to obtain adequate images with SPECT from all phases of the cardiac cycle with the help of ECG gating. In the following years, further technical progress in computing and introduction of multidetector cameras, with ever increasing calculation speed of computer systems, made G-SPECT a particularly attractive research and clinical decision-making tool. With the progress and significant improvement of hardware came improvements in dedicated software. G-SPECT-focused software packages allowed automated or semi-automated quantification of parameters of both myocardial perfusion and function; those were widely introduced into routine clinical practice. Among many others, software developed by Cedars-Sinai Medical Center (QGS–quantitative gated SPECT), Emory University (Atlanta, GA), Stanford University, University of Michigan (Ann Arbor, MI) and Yale University (New Haven, CT) should be highlighted. These computer programs provided users the ability to assess both LV perfusion and the systolic and diastolic myocardial function. Furthermore, additional software upgrades offered even more functions, such as regional wall thickening and motion evaluation, separate analysis of diastolic, systolic and ungated datasets. In addition, currently used systems offer evaluation of fusion studies (i.e. SPECT and G-SPECT with CMRI or angio-CT) offering not only further insights in cardiac function but also an important research tool in cell-based myocardial repair and regeneration therapies [11].
Current G-SPECT procedure
After tracer intravenous injection GSPECT acquisition using usually a dual-head gamma-camera is performed. During each acquisition 8, 16, or recently even 32 (depending on the equipment used) projection images are acquired at each projection angle. Each of the acquired images corresponds to a specific portion of the cardiac cycle, through the ECG gating. The ECG-gating hardware is connected to the acquisition computer that controls the gantry, so that all the data corresponding to each frame are automatically sorted by the gamma camera into the appropriate image matrix. All projection images acquired for a given interval can then be processed into a SPECT using filtered backprojection or iterative reconstruction techniques, and volumes relative to the various GSPECT intervals can be displayed in four-dimensional format (x, y, z, and time), allowing highly precise assessment of cardiac function. In practice, during GSPECT acquisition and post-processing of the acquired data, we are given two datasets – a standard SPECT dataset which allows perfusion evaluation and a larger gated SPECT dataset for function evaluation.
Clinical and research value of G-SPECT: perfusion and regional and global LV function
Assessment of ventricular function by means of GSPECT has added to perfusion assessments in clinical risk stratification. Nowadays there is ample evidence that the function variables from G-SPECT provide additional useful clinical information along with that on myocardial perfusion [12, 13]. Most importantly, it has been demonstrated that the added assessment of regional function by means of G-SPECT (Figures 1, 2) significantly improves identification of patients with all forms of coronary artery disease (CAD), especially those with multi-vessel disease and enables precision follow-up, offering a unique value in clinical monitoring and monitoring of the efficacy of novel therapies.
Figure 1
Evolution of G-SPECT myocardial perfusion and LV contractility in acute (revascularized) ischaemia. A – (baseline): G-SPECT myocardial bullseye images (perfusion, motion, and thickening) obtained in a 55-year-old man 5 days after LAD acute occlusion-related STEMI treated with primary PCI, showing a severe perfusion defect in a antero-septal, anteral and antero-lateral wall of the left ventricle as well as in the apex accompanied by a significant reduction in LV motion/contractility and thickening (left panel). In this patient, the baseline LVEF was 24% with EDV of 261 ml, ESV of 199 ml, and stroke volume (SV) of 62 ml. The LV volume and filling curves (right panel) show a significantly reduced overall LV contractility. While part of the LV contractility reduction is likely to be neurogenic (stunning) [30, 31], the disruption in the filling curve (“camel’s hump”) may indicate a forming aneurysm of the LV apex. B – (6-month follow-up): Bullseye images indicate improvement in LV perfusion, motion/contractility and thickening (left panel). EF increased to 37% with a reduction of EDV to 203 ml, ESV reduction to 128 ml, and SV increase to 74 ml. One can notice an improvement of both LV volume and filling curve (right panel). With the stunning mechanism of contractility impairment no longer playing a role [30, 31] , the improvement of contractility results from the effect of revascularization (note improved perfusion) combined with the effect of medical therapy. Any potential effect an investigational treatment on top of those 2, requires a rigorous evaluation in a double-blinded, sham/placebo-controlled study
ESV – end-systolic volume, EDV – end-diastolic volume, LAD – left anterior descending, SV – stroke volume.
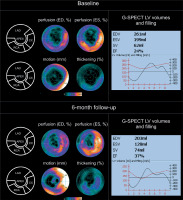
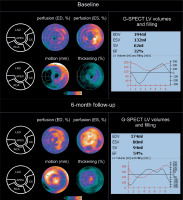
Figure 2
Evolution of G-SPECT myocardial perfusion and LV contractility in subacute (revascularized) ischaemia. A – (baseline): G-SPECT myocardial bullseye images obtained in 62-year-old female with subacute myocardial ischemia in 2-vessel coronary artery disease (primary PCI RCA revascularization and LAD revascularization 2 weeks later) demonstrate a significant perfusion defect in the apex and septum regions of the left ventricle accompanied by a significantly decreased in LV motion/contractility and thickening (left panel). One can notice a severe hypoperfusion in the affected areas, but still low tracer uptake indicates viable – most likely hibernating – myocardium in the LAD territory) with LV dilatation (right panel). Baseline LVEF was 32%, with EDV of 194 ml, ESV of 132 ml and SV of 62 ml. The LV volume and filling curves show reduced LV global contractility (right panel). B – (6-month follow-up): Bullseye images indicate improvement in LV perfusion, motion/contractility and thickening – that are more pronounced in the LAD territory (left panel). The results of this study further support the initial diagnosis of hibernating, hypoperfused myocardium at baseline in the LAD territory (angiographically tight proximal LAD stenosis, significant haemodynamically). One can notice improved perfusion in the antero-septal area with a profound improvement in wall motion in this area at 6-months. Also the dilation of LV observed at baseline (consistent with the initial contributory role of the stunning/neurogenic mechanism) [30, 31] is now gone, with LVEF increase to 54%, overall decrease in LV volumes (EDV of 174 ml, ESV of 80 ml) and SV increase to 94 ml (right panel)
ESV – end-systolic volume, EDV – end-diastolic volume, LAD – left anterior descending, PCI – percutaneous coronary intervention, RCA – right coronary arthery, SV – stroke volume.
There is ample evidence on the role of G-SPECT–determined LVEF, end-diastolic (EDV) and end-systolic (ESV) volumes in risk stratification. Evaluation of LV ESV provides added information over LVEF alone for prediction of cardiac death [14]. Perfusion deficits are stronger predictors of MI occurrence than contractility impairments [14–16].
Importantly, G-SPECT-determined LVEF is not subject to observer-dependent variability. For example, it is a reliable tool for evaluation of patients after STEMI with aneurysm. Wei et al. evaluated 96 patients with low LVEF (32 ±9%) diagnosed by cMRI (used as a reference), G-SPECT and G-PET. Global volume and contractility parameters, including EDV, ESV, and LVEF, were calculated using QGS software. With cMRI as a reference, LVEF was underestimated by G-SPECT but overestimated by G-PET (p < 0.001 for both) [17]. However, in general both methods proved to be reliable and correlated well with cMRI measurements [17]. LVEF measured by GSPECT and G-PET was affected by the akinetic/dyskinetic segments with absent wall thickening [17]. Importantly, the choice of software used to process G-SPECT data does not seem to affect the obtained results. Yao et al. [18] compared the accuracy of G-SPECT and G-PET in the assessment of EDVs, ESVs and LVEF among patients with prior myocardial infarction. The authors used different software packages (Quantitative Gated SPECT, QGS; Emory Cardiac Toolbox, ECTB; and 4D-MSPECT, 4DM), obtaining comparable results in all cases [18].
In CAD patients with progression toward chronic ischaemic heart failure (CIHF), development of cardiomyopathy or LV aneurysm, the phenomenon of left ventricular systole dyssynchronization is very often observed. With the occurrence of dyssynchrony, many diagnostic imaging methods – but not G-SPECT – fail to yield reliable results. Cho et al. [19] studied 109 acute MI patients with > 50% stenosis in at least one non-culprit artery who underwent GSPECT and were successfully revascularized. In addition to standard GSPECT evaluation, parameters related to functional dyssynchrony (such as the phase standard deviation, PSD; and phase histogram bandwidth, phase histogram bandwidth (PHB), were measured). The patients were followed for a median of 26 months after MI, for occurrence of major adverse cardiac events (MACE) (all-cause death, unplanned hospitalization due to heart failure and severe ventricular arrhythmias). MACE occurred in 22 (20%) patients, with both PSD and PBW significantly higher in patients with MACE compared to those without. The authors concluded that left ventricular mechanical dyssynchrony parameters and G-SPECT added prognostic value in acute MI with multivessel disease [19]. Similar conclusions came from the study by Fudim et al. [20], who analysed 1310 patients with at least 1 major epicardial obstruction ≥ 50%. G-SPECT dyssynchrony was assessed using Emory Cardiac Toolbox software. Systolic and diastolic left ventricular dyssynchrony was associated with adverse outcomes. Particularly important was the finding that diastolic dyssynchrony appeared to provide incremental predictive value to clinical history, electrical dyssynchrony, and left ventricular function [20, 21]. Specifically, systolic and diastolic left ventricular contraction synchrony disorders were associated with adverse outcomes, with the authors concluding that diastolic dyssynchrony appears to provide incremental predictive value to clinical history followed by changes in electrical synchrony, and left ventricular function.
In the case of ischaemic cardiomyopathy (ICM), which is considered an end-stage form of CAD, G-SPECT evaluation provides clinically important information. In the study performed by Candell-Riera et al. [22] 167 patients with ICM underwent rest myocardial perfusion G-SPECT. During an average follow-up of 2.3 ±1.2 years, cardiac mortality (CM) was 17.4%. Independent predictors of CM in rest G-SPECT were myocardial viability parameters (hazard risk (HR) 5.1; 95% CI: 1.2–21.4, p = 0.027). Interestingly, coronary angiography variables, evaluated in a subset of 111 patients, did not significantly modify the prognostic value of non-invasive testing with G-SPECT [22]. Studies performed in patients with dilated cardiomyopathy confirmed the similarity of results obtained with G-SPECT and cMRI [23–25]. Fundamental strengths and limitations of SPECT imaging in relation to cMRI imaging are presented in Table I [26–29].
Table I
Fundamental strengths and limitations of SPECT and MRI in assessing myocardial perfusion and LV volumes and ejection fraction [26–29]
Examples of the value of G-SPECT in tracking evolution of myocardial perfusion and global and regional contractility are presented in Figures 1 and 2 [30, 31].
Accuracy and reproducibility of SPECT: the pillars of its research value
G-SPECT offers automated (i.e. not requiring manual edge delineation) determination of LVEF, providing extremely high (up to 99%) reproducibility in LVEF measurements [32]. Similarly high (99%) reproducibility has been observed for G-SPECT perfusion assessment [33]. Recent analysis over different sites (10 centres in comparison to the core lab) of a diverse sample of patients with chronic ischaemic heart failure showed no significant differences in LV end-systolic volume, LV end-diastolic volume, LVEF, and percentage of left ventricle non-viable with good agreement in Bland-Altman plots, corroborating the strength of gated myocardial perfusion scintigraphy as a research tool with results applicable to routine clinical practice [34]. These findings enable the G-SPECT measures of LV contractility and perfusion to be used – with a scientific rigor applicable to clinical practice – as endpoints in randomized clinical trials comparing different revascularization strategies in patients with chronic ischaemic heart disease (including those with reduced LVEF) [35–37] and in patients with heart failure subjected to cell therapies [38, 39].








