Introduction
Primary percutaneous coronary intervention (PPCI) is an effective method for the clinical treatment of acute ST-segment elevation myocardial infarction (STEMI). In real-world settings, emergency coronary intervention is usually performed in patients with time from symptom onset < 12 hours. However, for patients who miss the optimal time window, the optimal management of these patients is still under debate. A study showed that for STEMI patients presenting between 12 and 48 hours after symptom onset, coronary revascularization is associated with better short- and long-term clinical outcomes than conservative treatment [1]. However, the optimal PCI timing affording maximal clinical benefits to patients with STEMI remains controversial. Although the guidelines recommend that for patients with time from symptom onset > 12 hours, the PPCI strategy is indicated in the presence of ongoing symptoms suggestive of ischemia, hemodynamic instability, or life-threatening arrhythmias, it is not always the case. It is amazing that we have so little data on the optimal reperfusion timing of such patients with STEMI presenting later than 12 hours from symptom onset. Important questions have to be addressed, but unfortunately, answers are not available for all of them. Available trials reporting outcomes of delayed PCI time frames after STEMI lack any consensus on the best reperfusion strategy [2]. Older adults are a high-risk group whose management requires a multidimensional clinical approach. The management of STEMI in the older population is challenging because of the high prevalence of comorbidities, atypical symptoms, delayed presentation, and delayed diagnosis. Despite the proven benefits of revascularization, the optimal therapy of STEMI in the older population needs to be ascertained individually [3]. Studies have shown that in the non-ST-elevation acute coronary syndrome (NSTE-ACS) patients, the early invasion strategy does not independently affect the prognosis [4], and compared with the delayed invasion strategy, the early invasion strategy cannot reduce the mortality [5].
Aim
In this study, we investigated the long-term outcome of PCI at different times in the elderly with acute STEMI with time from symptom onset > 12 hours.
Material and methods
Study population
This was a single-center prospective randomized controlled study. Three hundred and twenty elderly STEMI patients with onset time of more than 12 hours receiving PCI between July 2021 to July 2022 were enrolled in this study. Patients were partitioned according to Killip grading, and patients in each district were randomly divided into 4 groups: group 1 (immediate invasive strategy, PCI < 24 hours after symptoms onset, n = 80), group 2 (early invasive strategy, 24–< 72 hours, n = 80), group 3 (delayed invasive strategy, 72–< 168 hours, n = 80), and group 4 (late PCI group, ≥ 168 hours, n = 80). Baseline clinical variables, including patient demographics, medical history, laboratory diagnostic data and angiographic results, were obtained from the department’s electronic database. Patients were included if they met at least 2 of the following criteria for STEMI (characteristic severe chest pain lasting ≥ 30 minutes, electrocardiographic changes or elevation of cardiac serum biomarkers) and if they experienced continuous ongoing symptoms or signs of ischemia (persistent/recurrent symptoms of myocardial ischemia or persistent ST-segment elevation/re-elevation on electrocardiography). Patients were excluded from the study for the following: (1) younger than 60 years of age; (2) previous fibrinolytic treatment; or (3) lack of informed consent; or (4) treatment at a facility incapable of coronary angiography, angioplasty or stenting or (5) hemodynamic instability requiring emergency revascularization.
We followed the Declaration of Helsinki and were approved by the ethics committee of the Affiliated Hospital of Hebei University (HDFYLL-KY-2023-080). The Chinese clinical trial registration number is ChiCRT2300072737. Independent clinical research associates observed the trial and accumulated data.
Percutaneous coronary intervention and medical treatment
All patients received 300 mg of aspirin and 300 clopidogrel before PCI. Common heparin (100 IU/kg) was given before or during PCI to maintain the activated clotting time at 250 to 300 seconds. After PCI, 100 mg of aspirin and 75 mg of clopidogrel once daily, or 90 mg of ticagrelor twice daily, was prescribed as a maintenance dose for at least 1 year. The selection of PCI timing, medications, mode of vascular access, use of glycoprotein IIb/IIIa inhibitors, coronary stenting, and thrombus aspiration was left to the discretion of the attending physicians.
Study definitions and clinical outcomes
A successful PCI was defined as residual stenosis of < 30% and thrombolysis in myocardial infarction (TIMI) flow grade 3 in the infarct-related artery.
The primary study end points were the 12-month cardiac mortality, nonfatal myocardial infarction, target-vessel revascularization, and heart failure-related rehospitalization.
Statistical analysis
Continuous variables are presented as means ± standard deviations or as medians with interquartile ranges, and were compared using the unpaired t test or the Mann-Whitney rank sum test. Discrete variables are expressed as counts with percentages and were compared with the aid of Pearson’s c2 test or Fisher’s exact test. The main endpoint events were compared pairwise between four groups, using the Z test after the c2 test. We constructed Kaplan-Meier curves to compare mortalities in those who underwent PCI at different times. Differences were assessed with the aid of the log-rank test. Cox’s proportional hazards regression modeling (with adjustment for covariates) was used to assess clinical outcomes. The following variables were included in multivariate Cox’s regression analysis: gender, smoking history, hypertension, family history of coronary heart disease, diabetes. All analyses were 2-tailed, and a p-value < 0.05 was considered to reflect significance. Statistical analyses were performed using SPSS V.22.0 (IBM Corp.) and R software version 4.2.0.
Results
Baseline characteristics
The baseline clinical characteristics are summarized in Table I. There were no significant differences among the four groups.
Table I
Baseline clinical characteristics
Table II shows the angiographic and surgical characteristics of all patients. We found no significant differences in the severity of the coronary lesions, pre-PCI TIMI flow grade 0 in the infarct-related artery, number of implanted stents and the proportion of thrombus aspiration among the four groups. Pro-PCI TIMI flow grade 3 differed among the four groups, being more common in groups 3 and group 4 (82.5 vs. 88.6 vs. 93.8 vs. 96.3%, p = 0.017).
Table II
Procedural characteristics
| Parameter | < 24 h (Group 1, n = 80) | 24–< 72 h (Group 2, n = 80) | 72–< 168 h (Group 3, n = 80) | ≥ 168 h (Group 4, n = 80) | P-value |
|---|---|---|---|---|---|
| Infarct-related coronary artery: | 0.676 | ||||
| Left main | 6 (7.5%) | 7 (8.9%) | 8 (9.9%) | 5 (6.3%) | |
| Left-anterior descending | 27 (33.8%) | 33 (41.8%) | 29 (35.8%) | 30 (37.5%) | |
| Left circumflex | 5 (6.3%) | 2 (2.5%) | 5 (6.2%) | 4 (5.0%) | |
| Right | 10 (12.5%) | 9 (11.4%) | 8 (9.9%) | 14 (17.5%) | |
| Multivessel coronary disease | 32 (40.0%) | 28 (35.4%) | 31 (38.3%) | 27 (33.8%) | |
| Pre-PCI TIMI flow grade 0 | 36 (45.0%) | 28 (35.4%) | 29 (35.8%) | 22 (27.5%) | 0.149 |
| Number of stents: | 0.826 | ||||
| 0 | 6 (7.5%) | 2 (2.5%) | 5 (6.2%) | 3 (3.8%) | |
| 1–2 | 55 (68.8%) | 59 (74.7%) | 54 (66.7%) | 62 (77.5%) | |
| ≥ 3 | 19 (23.8%) | 18 (22.8%) | 22 (27.2%) | 15 (18.8%) | |
| Pro-PCI TIMI flow grade 3 | 66 (82.5%)a | 70 (88.6%)a,b | 76 (93.8%)b | 77 (96.3%)b | 0.017 |
| Thrombus aspiration | 37 (46.3%) | 30 (38.0%) | 31 (38.3%) | 23 (28.8%) | 0.156 |
A total of 15 (4.6%) patients in the four groups had cardiac death during the 12-month follow-up period (Table III). During follow-up, among the four groups, heart failure-related rehospitalization was higher in group 1 than in the other groups (18.8 vs. 5.1 vs. 7.4 vs. 6.3%, p = 0.010). The incidence rates of cardiac death, target vessel revascularization and non-fatal myocardial infarction were comparable among the four groups (Table III). Compared with group 1, group 2, group 3 and group 4 had lower heart failure-related rehospitalization (HR = 0.250, 95% CI: 0.083–0.753, p = 0.014) (HR = 0.377, 95% CI: 0.146–0.971, p = 0.043) (HR = 0.320, 95% CI: 0.116–0.879, p = 0.027) (Table IV).
Table III
12-month primary study end points
| Parameter | < 24 h (Group 1, n = 80) | 24–< 72 h Group 2, n = 80) | 72–< 168 h (Group 3, n = 80) | ≥ 168 h (Group 4, n = 80) | P-value |
|---|---|---|---|---|---|
| Target-vessel revascularization | 3 (3.8%) | 5 (6.3%) | 4 (4.9%) | 6 (7.5%) | 0.752 |
| Heart failure-related rehospitalization | 15 (18.8%)a | 4 (5.1%)b | 6 (7.4%)b | 5 (6.3%)b | 0.010 |
| Nonfatal myocardial infarction | 11 (13.8%) | 4 (5.1%) | 6 (7.3%) | 7 (8.8%) | 0.254 |
| Cardiac death | 5 (6.3%) | 4 (5.1%) | 3 (3.7%) | 3 (3.8%) | 0.855 |
Table IV
Single factor analysis of heart failure-related rehospitalization
| Items | B | SE | Wald | HR | P-value |
|---|---|---|---|---|---|
| Group 1 | Reference | ||||
| Group 2 | –1.387 | 0.563 | 6.075 | 0.250 (0.083, 0.753) | 0.014 |
| Group 3 | –0.976 | 0.483 | 4.084 | 0.377 (0.146, 0.971) | 0.043 |
| Group 4 | –1.141 | 0.516 | 4.878 | 0.320 (0.116, 0.879) | 0.027 |
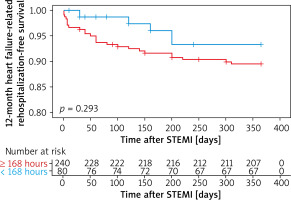
Kaplan-Meier survival curves showed that PCI within 24 hours increased the risk of 12-month heart failure-related rehospitalization compared with those who underwent later PCI (plog-rank < 0.01, Figure 1). Neither within 72 hours nor within 168 hours did 12-month heart failure-related rehospitalization differ compared with those who underwent PCI later (plog-rank = 0.132, Figure 2) (plog-rank = 0.293, Figure 3) (Tables V, VI).
Table V
Single factor Cox analysis of cardiac death
Table VI
Multifactor Cox analysis of cardiac death
Figure 1
Kaplan-Meier curves for 12-month heart failure-related rehospitalization-free survival in patients who received PCI < versus ≥ 24 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
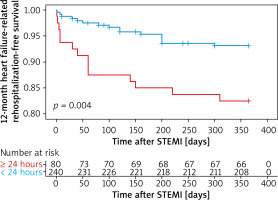
Figure 2
Kaplan-Meier curves for 12-month heart failure-related rehospitalization-free survival in patients who received PCI < versus ≥ 72 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
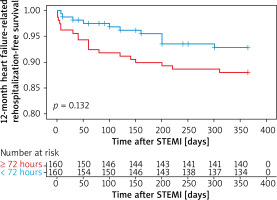
Figure 3
Kaplan-Meier curves for 12-month heart failure-related rehospitalization-free survival in patients who received PCI < versus ≥ 168 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
COX analysis of cardiac death showed that hypertension and Killip class were independent predictors of cardiac death.
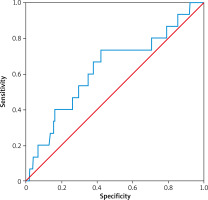
The area under the curve of LVEF for predicting cardiac death in patients with acute ST-segment elevation myocardial infarction was 0.613, the specificity was 0.680 and the sensitivity was 0.733. The optimum critical value was 48% (Figure 4, Table VII).
Table VII
ROC curve analysis of LVEF in cardiac death
| Item | AUC | Standard error | P-value | 95% CI | |
|---|---|---|---|---|---|
| LVEF | 0.628 | 0.080 | 0.094 | 0.472 | 0.784 |
A total of 54 (16.8%) patients in the four groups had atrial fibrillation, and the 12-month primary study end points were comparable among the four groups (Table VIII, Figures 5–7).
Table VIII
12-month primary study end points of patients with atrial fibrillation
Figure 5
Kaplan-Meier curves for 12-month heart failure-related rehospitalization-free survival (A), cardiac death-free survival (B), target-vessel revascularization-free survival (C), nonfatal myocardial infarction-free survival (D) in patients with atrial fibrillation who received PCI < versus ≥ 24 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
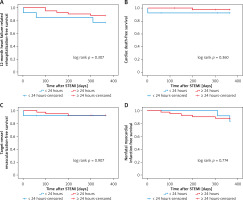
Figure 6
Kaplan-Meier curves for target-vessel revascularization-free survival (A), cardiac death-free survival (B), heart failure-related rehospitalization-free survival (C), nonfatal myocardial infarction-free survival (D) in patients with atrial fibrillation who received PCI < versus ≥ 72 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
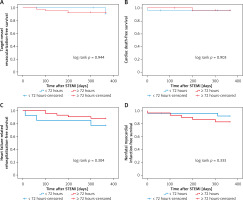
Figure 7
Kaplan-Meier curves for heart failure-related rehospitalization-free survival (A), nonfatal myocardial infarction-free survival (B), target-vessel revascularization-free survival (C), cardiac death-free survival (D) in patients with atrial fibrillation who received PCI < versus ≥ 168 hours after symptoms onset
STEMI – ST-segment elevation myocardial infarction.
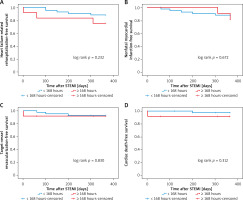
Discussion
Along with the improvement in life expectancy across the world, elderly people with acute STEMI with time from symptom onset > 12 hours are common. They may be misdiagnosed due to atypical symptoms and miss the optimal time for coronary artery opening, but there is no consensus on whether an immediate invasive strategy is also beneficial for those patients [6, 7]. A study involved 6134 NSTEMI patients undergoing PCI from the Korea Acute Myocardial Infarction Registry, which showed that for NSTEMI patients, immediate PCI had higher 12-month rates of MI and death [8]. In the meta-analysis by Katritsis et al. [9], there were no statistically significant differences in mortality or myocardial infarction between early coronary angiography and delayed coronary angiography in acute coronary syndromes without ST-segment elevation (NSTE-ACS). The ABOARD trial showed that immediate PCI was associated with an increased rate of MI in comparison with a 24–48 hours deferred strategy for high-risk patients with non-refractory NSTE-ACS [10, 11]. Myocardial viability has been documented > 12 hours after coronary occlusion, preserved by residual blood flow to vulnerable areas [12]. Moreover, the viability of stunned myocardium has been confirmed days to weeks following acute myocardial infarction (MI), suggesting that revascularization may prevent overt necrosis or apoptosis in dormant myocardium [13]. Data from the BRAVE-2 trial indicate that myocardial salvage is an important mechanism by which mechanical reperfusion may benefit patients with STEMI presenting ≥ 12 hours after symptom onset [14]. In a recent meta-analysis of late percutaneous coronary intervention (PCI) for acute MI (≥ 12 hours to 60 days after symptom onset, 12-day median symptom duration), PCI appeared to improve cardiac function and survival [15, 16]. Along with an ongoing revolution in current clinical therapeutics lowering the rate of perioperative complications, practicing physicians are then apt to shorten this waiting time under amenable conditions. However, in the study, immediate invasive strategy did not reduce the incidence of 12-month cardiac mortality, heart failure re-hospitalization, target vessel revascularization, and nonfatal myocardial infarction in these patients. During follow-up, heart failure-related rehospitalization was higher in group 1 than in the other groups (18.8 vs. 5.1 vs. 7.4 vs. 6.3%, p = 0.010). Compared with group 1, group 2, group 3 and group 4 had a lower heart failure-related rehospitalization (HR = 0.250, 95% CI: 0.083–0.753, p = 0.014) (HR = 0.377, 95% CI: 0.146–0.971, p = 0.043) (HR = 0.320, 95% CI: 0.116–0.879, p = 0.027) (Table IV). Kaplan-Meier survival curves showed that PCI within 24 hours increased the risk of 12-month cardiac mortality compared with those who underwent later PCI (plog-rank < 0.01, Figure 1). The possible reasons are as follows: (1) PCI triggered further myocardial depression. On one hand, mechanical stress from hemodynamic perturbations or interventional manipulation of epicardial coronary atherosclerotic plaques with inflammatory destabilization can release particulate debris, thrombotic material and soluble substances into the coronary circulation. The physical material obstructs the coronary microcirculation, whereas the soluble substances induce endothelial dysfunction and facilitate vasoconstriction. Coronary microvascular obstruction and dysfunction result in patchy microinfarcts accompanied by an inflammatory reaction, both of which contribute to progressive myocardial contractile dysfunction [17–20]. Various thrombus extraction devices have been developed to remove thrombus during STEMI, while in clinical studies, the benefit of protection devices to retrieve atherothrombotic debris during percutaneous coronary interventions has been modest [21]. In two large trials powered for clinical end points, no reduction in 1-year mortality or other adverse clinical events was observed with the use of this strategy. Moreover, one of these trials showed a marginally increased risk of stroke [22]. In our study, the immediate PCI group had a heavy thrombus load, which was likely to lead to myocardial microcirculation disturbance. On the other hand, the poorer clinical outcomes in immediate invasion strategy groups have been proposed to reflect further deterioration of heart function caused by ischemia/reperfusion injury, the no-reflow (NR) phenomenon that develops during PCI, and reduced heart function [23]. In our study, the immediate PCI group had a heavy thrombus load, which was likely to lead to the no-reflow phenomenon [24, 25]. Approximately a third of patients treated by PCI for ST-segment elevation myocardial infarction will suffer from coronary no-reflow [25], a condition characterized by poor myocardial perfusion despite patent epicardial arteries. The presence of NR has a significant impact on clinical outcomes including left ventricular dysfunction, heart failure and death [25]. (2) Spontaneous coronary reperfusion of the infarct-related artery occurs in 7–27% of patients experiencing acute ST elevation myocardial infarction [26–30]. A study showed that early PCI (< 24 hours from pain onset) in patients with STEMI due to spontaneous coronary reperfusion seemed to result in an excess of early major adverse cardiovascular events (MACE) without any long-term advantage [31]. Thrombus autolysis may be more likely to occur in patients undergoing later PCI. An article published in the New England Journal of Medicine (NEJM) showed that when coronary angiography was completed within 6 hours prior to a coronary event, almost all the arteries associated with infarction were blocked by new thrombi. When coronary angiography was delayed 24 hours, the number of blocked vessels decreased, which suggests that there is a spontaneous thrombolytic process; Ellis et al. also described the same phenomenon in an experimental model of myocardial infarction [32]. Later PCI is superior to emergency PCI in improving postoperative myocardial perfusion in STEMI patients with high thrombotic load, and can effectively reduce the MACE [33]. (3) Changes in clot structure are of key interest due to associations with risk of myocardial infarction. However, studies on the structure and components of thrombi formed in vivo remain limited. Thrombus composition from patients with myocardial infarction is influenced by ischemic time [34]. Therefore ischemia time is a key determinant of thrombus composition. Silvain et al. [35, 36] demonstrated that as ischemic time increased the amount of fibrin increased while platelets decreased in STEMI thrombi. STEMI thrombi (n = 40) retrieved over 12 hours after the onset of symptoms showed more fibrin than thrombi retrieved within 3 hours. Correspondingly, RBCs decreased over time [37]. STEMI thrombi (n = 65) retrieved > 6 hours after onset of symptoms showed a more compact fibrin network than thrombi retrieved < 3 hours. With increasing time after symptom onset, platelet numbers decreased [38]. Platelets triggered thrombus formation, which occurs more in “fresh” clots, while fibrin enhances the overall clot structure and plays an important role later. Taken together, these findings indicate that thrombus composition may change over time after initial vessel occlusion in STEMI [39, 40]. Such changes in thrombus composition may have important implications for the selection of intervention timing [41, 42]. Further research is required to understand the mechanisms through which thrombi change structure, for example, through thrombus component reorganization or clotting-lysis cycles.
Notably, only part of our study population underwent PCI within 24 hours, which suggested that many cardiologists hesitate to perform immediate invasive coronary angiography in elderly acute STEMI patients with time from symptom onset > 12 hours in real-world clinical practice, probably because they fear development of the abovementioned problems.
Conclusions
For acute STEMI patients who missed the optimal time of PCI, immediate PCI did not reduce adverse clinical outcomes. It is possible that delayed PCI may be safer, after a “cooling down” period, potential thrombus resolution and plaque stabilization.
Our current study had several limitations. First, we enrolled a relatively small number of patients. Second, the 12-month follow-up period was insufficient to evaluate long-term adverse events.