Introduction
Clear cell renal cell carcinoma (ccRCC) exhibits a tendency for intravascular growth, and it occasionally presents with neoplastic thrombus expanding into the renal vein or even further to vena cava [1]. This phenomenon correlates with a higher risk of metastatic spread and inferior overall survival (OS), but multiple additional factors influence the final outcomes in this clinical scenario [2]. The risk stratification is crucial to personalise the pre – and/or post-surgical management of these patients [3]. Recent advances in the treatment of renal cancer have initiated changes in the landscape of the available modalities [4]. As novel immunotherapy has already proven its place in this setting, there is a growing need for biomarkers to be implemented in the phase of patients’ qualification for systemic treatment or monitoring during follow-up [5]. Interestingly, the primary tumour and its thrombus may display distinct biological features and microenvironments. A recent multicentre study showed that novel immune markers (PD-L1, VISTA, TOX) expressed by tumour cells (TC) and tumour-associated immune cells (TAIC) in the primary renal tumour mass and venous thrombus are associated with OS [6, 7]. Moreover, their expression correlates with markers of systemic inflammation derived from complete blood count, which are potentially useful prognostic factors in RCC with venous thrombus. VISTA [6] represents the next generation of immune checkpoints inhibitors [8]. Interestingly, up-regulation of VISTA on cancer cells may be attributed to the immunotherapy used, being the sign of immune escape mechanism [9]. The abundance of VISTA-positive TAIC in venous tumour thrombus corresponds with poorer survival in ccRCC [6].
VISTA is thought to be an acidic pH-selective target for P-selectin glycoprotein ligand-1 (PSGL-1) [10]. P-selectin glycoprotein ligand-1 major function is based on the regulative functions of leukocyte trafficking and adhesive features of the endothelium [11]. As a promising candidate for becoming a future biomarker, PSGL-1 is commonly expressed by myeloid cells and activated lymphocytes T, and, occasionally, TC [12]. Hence, we hypothesised that its expression may differ within various compartments, i.e. the primary tumour and tumour thrombus, and may be of prognostic significance. In the current study, we investigated the clinicopathological correlates and prognostic value of PSGL-1 in ccRCC with venous tumour thrombus.
Material and methods
Patient cohort
Eighty-two patients with primary ccRCC and venous tumour thrombus treated surgically upfront with nephrectomy with/without cavotomy and subsequent thrombectomy in the years 2012–2019 in 2 tertiary urological departments were enrolled. No additional treatment was managed prior to the definite surgery. Surgical procedures were performed in a standardised manner through laparotomy. We collected the following data from patients: age, gender, tumour stage based on computed tomography or magnetic resonance imaging scans of thorax, abdomen, and pelvis according to 2017 TNM (tumour-node-metastasis) classification system (AJCC version) [13], preoperative haematological data (number of neutrophils, platelets, lymphocytes, monocytes together with respective ratios: neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, platelet to lymphocyte ratio) retrieved from local certified laboratories (FACS, Sysmex XM200, Sysmex Poland, Poland), the results of pathological examination, as well as the last follow-up and survival outcomes from local institutional databases. Telemedicine visits were established if necessary to incorporate any missing data.
The study was performed under local Ethics Committee approval AKBE/72/2021 of the Medical University of Warsaw. Informed consent was collected from all the patients involved in the study.
Tissue microarrays and immunohistochemistry
Haematoxylin and eosin-stained ccRCC specimens from the renal tumour mass and venous tumour thrombus were reviewed by 2 pathologists (R.P. and M.K.). All cases were examined for the presence of TAIC, i.e. tumoral/peritumoral lymphocytes and macrophages. Representative areas containing cancer cells and TAIC were selected. Tissue microarrays (TMA), containing representative samples of the primary tumour mass and venous thrombus of 82 ccRCC cases, were created using 1.5 mm needles, with 3 cores sampled from both the venous thrombus (2 peripheral, one central) and the renal tumour. Smaller thrombi had fewer cores. Control samples from tonsil, placenta, and liver were included. Tissue microarrays were sliced into 5 µm sections for immunohistochemistry [6, 7]. Subsequently, obtained TMA were stained with anti-PSGL-1 antibody (clone KPL-1, mouse monoclonal, dilution 1 : 200, Sigma Aldrich), anti-VISTA (clone D5L5T, dilution 1 : 300, Cell Signaling), and/or anti-PD-L1 antibody – 22c3 mouse monoclonal, dilution 1:50 (DAKO, Agilent, CA, USA). PSGL-1 expression was assessed separately in TC and TAIC in both tumour compartments. Cores containing tonsil and placenta tissue served as positive controls, whereas liver samples served as negative controls [14]. The membranous and cytoplasmatic reaction were considered positive, and the percentage of positive cells of each type was scored by 2 pathologists with experience in uropathology (RP and MK). The differences in scoring between the 2 patho- logists were noted, and the final consensus scores were obtained after discussion.
Statistics
Statistical analyses were performed using the Statistica 13.3 (TIBCO, Palo Alto, CA, USA; licensed to the Medical University of Gdańsk) and R statistical environment [15]. Associations between categorical variables were assessed with the χ2 test or Fisher’s exact test when applicable. Continuous variables were analysed using the Wilcoxon test, Kruskal-Wallis test, or Spearman correlation, as appropriate. Kaplan-Meier curves were plotted using the “survminer” package and compared with the log-rank test [16]. Hazard ratios were calculated with the Cox proportional hazard regression. All tests were considered statistically significant as p ≤ 0.05.
Results
Basic characteristics of the studied cohort
Four cases were excluded from the analysis due to the loss of cores in TMA, leaving 78 cases for further analysis. In this group median follow-up was 29 months (Table 1). The 2-year OS during follow-up reached 74.6%. The summary of the cohort is presented in Table 1.
Table 1
Basic characteristics of the study group
The expression of P-selectin glycoprotein ligand-1 on tumour cells and tumour-associated immune cells in different compartments
Representative examples of PSGL-1 staining in primary RCC and venous tumor thrombus were presented in Figure 1. In primary tumors, PSGL-1 was expressed on TC and TAIC in 29 (37.2%) and 61 (78.2%) cases, respectively. In venous thrombus, any expression of PSGL-1 was observed on TC and TAIC in 46 (59%) and 38 (48.7%) cases, respectively (Fig. 2, Table 2). Expression of PSGL-1 on TC was significantly more common in venous thrombus (p < 0.0001). On the other hand, the percentage of PSGL-1 expressing TAIC was significantly higher in the primary tumour (p < 0.0001) (Table 2).
Fig. 1
Representative examples of P-selectin glycoprotein ligand-1 (PSGL-1) staining in immune cells infiltrating clear cell renal cell carcinoma. PSGL-1-negative TC and a few (5%) -positive tumour-associated immune cells (TAIC) in thrombus (A), negative TC and 90% positive TAIC in tumour (B), strongly positive TC and TAIC in thrombus (C), moderately positive TC and negative TAIC in tumour (D) PSGL-1 – P-selectin glycoprotein ligand-1, TAIC – tumour-associated immune cells, TC – tumour cells
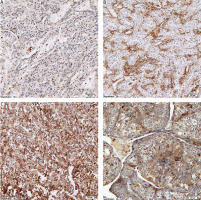
Fig. 2
Expression of P-selectin glycoprotein ligand-1 on tumourassociated immune cells in venous tumour thrombus and primary tumour (renal cell carcinoma) PSGL-1 – P-selectin glycoprotein ligand-1, TAIC – tumour-associated immune cells The associations were estimated with paired Wilcoxon test.
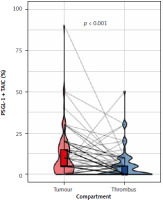
Table 2
The comparison of P-selectin glycoprotein ligand-1 expression between the primary tumour and venous thrombus, concerning the type of PD-L1 expressing cells
| PSGL-1 | Primary tumour | Venous thrombus | ||
|---|---|---|---|---|
| TC | TAIC | TC | TAIC | |
| Positive, n (%) | 29 (37.2) | 61 (78.2) | 46 (59) | 38 (48.7) |
| Negative, n (%) | 49 (62.8) | 17 (21.8) | 31 (39.7) | 39 (50) |
| Missing, n (%) | 1 (1.3) | 1 (1.3) | ||
The association between P-selectin glycoprotein ligand-1 expression and clinicopathological variables
Several positive correlations were noted between PSGL-1 expression in different compartments/cells and clinicopathological findings (Table 3). Cancers expressing PSGL-1 on TC in tumour thrombus more commonly demonstrated, among others, high-grade histology (p = 0.017), whereas expression on TAIC correlated with the presence of necrosis (p = 0.014). Recently, we focused on the expression of VISTA in TC and TAIC in RCC with tumour thrombus [6]. Because no expression of VISTA was found in TC, we continued analysis in TAIC and found positive correlation with the presence of VISTA and PSGL-1 on TAIC (Fig. 3).
Fig. 3
The correlation between VISTA and P-selectin glycoprotein ligand-1 expression on tumour-associated immune cells PSGL-1 – P-selectin glycoprotein ligand-1, TAIC – tumour-associated immune cells
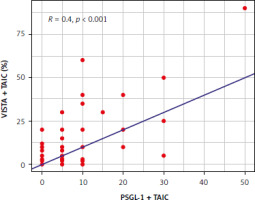
Table 3
The association between P-selectin glycoprotein ligand-1 expression in different compartment/cells and clinicopathological findings (all χ2, positive until stated otherwise)
Survival analysis
Patients with PSGL-1-positive TAIC in venous thrombus (Fig. 4 A) and PSGL-1-positive TC in the primary tumour (Fig. 4 B) had significantly shorter OS (respectively, HR 2.27, 95% CI: 1.06–4.84, p = 0.032, and HR 3.42, 95% CI: 1.45–8.05, p = 0.004, univariate Cox) (Table 4). Conversely, PSGL-1 expression on TC in thrombus and on TAIC in primary tumours had no significant impact on the oncological outcomes (univariate Cox) (Table 4). To further explore the prognostic impact of PSGL-1, we performed multivariate Cox regression analysis (Table 5), but PSGL-1 did not retain its prognostic value in the model controlled by nodal status, distant metastases, grade, and necrosis.
Fig. 4
Kaplan-Meier curves for overall survival according to P-selectin glycoprotein ligand-1 expression on tumour-associated immune cells in venous tumour thrombus (A) and tumour cells in primary tumour (B) neg – negative expression, pos – positive expression, PSGL-1 – P-selectin glycoprotein ligand-1, TAIC – tumour-associated immune cells, TC – tumour cells p-values were calculated with the log-rank test
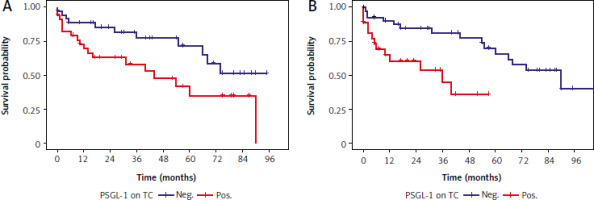
Table 4
Univariate Cox proportional hazard regression analysis of the association between – selectin glycoprotein ligand-1 expression in the primary tumour and venous tumour thrombus, concerning tumour-associated immune cells and tumour cells, and overall survival
| Feature | HR | p-value |
|---|---|---|
| PSGL1 TC PT (+) | 3.42 (1.45–8.05) | 0.004 |
| PSGL1 TAIC PT (+) | 0.73 (0.31–1.74) | ns |
| PSGL1 TC VT (+) | 1.12 (0.53–2.41) | ns |
| PSGL1 TAIC VT (+) | 2.27 (1.06–4.84) | 0.032 |
Table 5
Multivariate Cox proportional hazard regression analysis of the associations between P-selectin glycoprotein ligand-1 expression, clinicopathological variables (N, M, necrosis, grade), and overall survival
Discussion
In this study, we explored the expression of PSGL-1 in primary tumours and venous thrombi of ccRCC and its correlation with clinicopathological features and OS. We revealed significant differences in the expression of PSGL-1 between tumour compartments, with the higher prevalence of PSGL-1-positive TC in venous thrombi, and PSGL-1-positive TAIC in primary tumour.
Relatively more common expression of PSGL-1 on TC in venous thrombi may suggest its role in mediating TC interactions with platelets and the endothelium, which may facilitate metastatic dissemination [10]. This is consistent with previous studies indicating that PSGL-1, primarily known for its role in leukocyte trafficking and endothelial adhesion, may contribute to the metastatic potential of cancer cells by enhancing their adhesion properties under shear stress conditions found in the bloodstream [17]. In contrast, the study by Yamaoka et al. suggests that PSGL-1 plays a role in promoting immune cell infiltration and suppressing primary tumour growth in a murine model [18]. This discrepancy underscores the complex and context-dependent roles of PSGL-1 in tumour biology. Further research is needed to elucidate the mechanisms by which PSGL-1 influences tumour progression and to understand the differences observed between murine models and human cancers [18]. Interestingly, our analysis identified that PSGL-1 positivity in primary tumour TC and venous thrombi TAIC correlated with inferior OS. However, this prognostic significance did not retain significance in multivariate analysis controlled for by well-established prognostic factors (nodal status, distant metastases, tumour grade, necrosis).
The association of PSGL-1 expression with high-grade histology in both primary tumours and thrombi may further support its role in promoting tumour aggressiveness. Moreover, the presence of PSGL-1-positive TAIC in venous thrombi correlated with tumour necrosis, another adverse prognostic factor in ccRCC. These findings emphasise the complexity of the tumour microenvironment and the multifaceted role of PSGL-1 in general cancer biology. Another study supports our hypotheses based on the findings coming from the studies on cervical cancer, i.e. PSGL-1 mRNA expression was strongly related with the higher grade in the cervical high-grade squamous lesions. Despite this, it was found to be a good prognostic factor in cervical cancer [19]. It seems that specific tumour and organ microenvironment context may influence the prognostic significance of immune-related proteins like PSGL-1. Likewise, expression of PD-L1 shows divergent effects on outcomes in various tumours and organs.
The differential expression patterns of PSGL-1 also highlight the need for compartment-specific analysis when evaluating biomarkers in ccRCC. The distinct biological features and microenvironments of primary tumours and their associated thrombi necessitate a nuanced approach to biomarker assessment [20]. Our study suggests that PSGL-1 evaluation in venous thrombi, in addition to primary tumours, could provide a more comprehensive prognostic picture and potentially guide therapeutic stra- tegies.
Lastly, one should consider some of the limitations of our retrospective study when interpreting the results. The specific cohort that was analysed comprised predominantly patients with T3a stage with better expected outcomes when compared to more locally advanced cases. Additionally, no subanalyses of the populations of TAIC were available. The study was performed on TMA, and their assessment may not adequately reflect the whole tumour microenvironment.
Conclusions
In the current study we found that PSGL-1 was more commonly expressed by TC in the venous thrombus than in the main renal mass of ccRCC. It suggests that the acquisition of PSGL-1 expression in the tumour thrombus enables interactions of TC with platelets and endothelium. Then, PSGL-1 expression on TC was proven to have prognostic value only in the tumour compartment. Interestingly, the presence of PSGL-1-positive TAIC in venous thrombus was also associated with worse outcomes. Thus, the assessment of prognostic biomarkers in ccRCC should take into consideration the unique environment of various compartments.








