Introduction
The right minithoracotomy approach for aortic valve replacement offers reduced operative trauma, increased postoperative recovery, diminished complication rates, improved cosmetic result, and increased patient satisfaction [1].
The hybrid strategy to treat patients with aortic valve disease and coronary artery disease is based on a combination of cardiac surgery methods to replace the aortic valve, preferentially through a minimally invasive approach and interventional cardiology methods to revascularize coronary arteries by percutaneous coronary intervention (PCI). Both the sequence of interventions and strategy of the antiplatelet therapy are still a matter of debate.
Aim
We decided to study the results of this minimally invasive hybrid approach (RT-AVR/PCI) in comparison with conventional aortic valve replacement (AVR) and coronary artery bypass grafting (CABG) through median sternotomy (AVR/CABG).
Material and methods
The study was performed in accordance with the Declaration of Helsinki. Approval from the Institutional Review Board (Jagiellonian University of Krakow, Poland) was obtained, and a review of prospectively gathered data of patients who underwent aortic valve replacement surgery and coronary revascularization between August 2011 and December 2013 was performed. There were 86 patients who underwent hybrid minimally invasive aortic valve replacement surgery through right minithoracotomy and percutaneous coronary intervention (RT-AVR/PCI, study group), and 101 patients with aortic valve disease and coronary artery disease, for whom conventional aortic valve surgery and coronary artery bypass grafting through median sternotomy (AVR/CABG, control group) was performed during the study period. AVR/CABG surgery was performed on the basis of standard guidelines for AVR and CABG for patients with severe aortic valve disease and coronary artery disease [2, 3].
Patients who underwent the hybrid procedure consisted of 2 subgroups: group A: RT-AVR/PCI procedures during the same session (one-stage hybrid subgroup), and group B: RT-AVR was performed within 90 days after PCI (two-stage hybrid subgroup). Patients who underwent the hybrid procedure during the same interventional session were scheduled for hybrid RT-AVR/PCI following consultation by the heart team; they were elective patients including both higher-risk elderly patients and normal-surgical-risk patients where PCI was performed in the catheterization laboratory immediately after RT-AVR had been performed in the operating room. The one-stage hybrid RT-AVR/PCI was scheduled for patients on the basis of standard guidelines for AVR and PCI for patients with severe aortic valve disease and coronary artery disease. For patients who underwent one-stage hybrid RT-AVR/PCI, RT-AVR surgery was usually performed in a morning session, and immediately afterwards RT-AVR patients were transferred to the catheterization laboratory where PCI was performed.
The two-stage hybrid RT-AVR/PCI, in which PCI was done first and followed within 90 days by RT-AVR, was performed for patients having aortic valve disease and coronary artery disease, who presented acute coronary syndrome (ACS). In such situations, urgent PCI was performed to re-establish coronary perfusion, and aortic valve replacement was postponed to diminish the risk of surgery. Contraindications for RT-AVR surgery included right pleural cavity adhesions and ascending aorta located under the sternum, when it was positioned more to the left side of the thoracic cavity [4].
Preoperative transthoracic echocardiography (TTE) and coronary angiography were performed on all patients included in our study. Based on coronary angiography, patients were qualified for either PCI or CABG according to guidelines [5]. Both drug-eluting stents (DES) and bare metal stents (BMS) were implanted during PCI.
Preoperative data and in-hospital and post-discharge outcomes were collected from medical data records. Patients who had reoperative surgery of the aortic valve or coronary arteries were excluded from the study, as well as emergency patients with aortic valve endocarditis.
Anticoagulation and antiplatelet therapy protocol
In the RT-AVR/PCI hybrid group, antiplatelet therapy was applied. In group A, when RT-AVR was finished, loading doses of aspirin (300 mg) and clopidogrel (300 mg) were administered. This was followed by 75 mg of aspirin and 75 mg of clopidogrel given daily to all patients after PCI. Heparin was administered during RT-AVR to keep ACT above 400 s, and during PCI to keep ACT above 250 seconds. In group B, at the time of PCI, loading doses of aspirin (300 mg) and clopidogrel (300 mg) were given, which was followed by 75 mg of aspirin and 75 mg of clopidogrel given daily to all patients. When planning RT-AVR, it was our recommendation to stop clopidogrel at least 7 days before surgery. This recommendation was not strictly followed by referring doctors, so patients underwent RT-AVR being on single antiplatelet therapy, dual antiplatelet therapy, or no antiplatelet therapy.
Analysed clinical parameters and definitions of perioperative events
The primary endpoints of the analysis were mortality and morbidity parameters. Predicted hospital mortality was calculated based on EuroSCORE II algorithms for the preoperative calculation of risk for AVR [6]. It was calculated before surgery; for patients from the control group before AVR/CABG, for patients from the group A before RT-AVR/PCI, or before RT-AVR for group B. Hospital mortality was defined as death for any reason that occurred within 30 days of surgery or during the same hospitalisation period if it was longer than 30 days [1]. Postoperative myocardial infarction was defined as troponin I at a minimum level of 1.0 ng/ml within 24 h of surgery [7]. Renal failure was considered present when the baseline creatinine level was > 1.5 mg/dl [8]. Prolonged ventilation time was the use of mechanical lung ventilation for longer than 24 h after surgery; superficial wound infection was present when skin and subcutaneous tissue was infected, while deep wound infection affected bones (the sternum or ribs), frequently with bone instability, or deeper intrathoracic tissue.
Statistical analysis
Categorical variables were expressed as counts and percentages. Empirical distribution of continuous variables was described using mean and standard deviation (SD), and additionally described using median and quartiles. For continuous variables, statistical significance of differences between 2 independent groups was assessed using the Mann-Whitney test. For 2 categorical variables the Fisher exact test or the χ2 test was used. Associations between a binary dependent variable and multiple independent variables were assessed using a bias reduced logistic regression model and reported using odds ratios (OR) with 95% confidence intervals (CI). A p-value less than 0.05 was considered an indication of a statistically significant result. No adjustment for multiple comparisons was made. All statistical analyses were performed using R 3.0 [9].
Results
The study group consisted of 86 patients with a mean age of 69.0 ±8.9 years, who underwent RT-AVR/PCI hybrid surgery, and the control group consisted of 101 patients with a mean age of 68.0 ± 9.4 years, who underwent AVR/CABG surgery between August 2011 and December 2013. The preoperative clinical status of patients is presented in Table I. There was no significant difference between groups regarding preoperative patient characteristics. All patients with aortic valve disease and coronary artery disease who presented unstable angina (UA) symptoms or with recognised non-ST elevation myocardial infarction (NSTEMI) or ST elevation myocardial infarction (STEMI) were treated with the RT-AVR/PCI hybrid approach; there was no conventional surgery (AVR/CABG) performed in the case of aortic valve disease and acute ACS.
Table I
Clinical status of patients before AVR/CABG or RT-AVR/PCI
[i] Continuous variables are described by median [1st and 3rd quartile], categorical variables are shown as a percentage, n – number of patients, EF – ejection fraction, COPD – chronic obstructive pulmonary disease, NYHA – New York Heart Association, UA – unstable angina, NSTEMI – non-ST elevation myocardial infarction, STEMI – ST elevation myocardial infarction, CCS – Canadian Cardiovascular Society classification. LAD – left anterior descending coronary artery, Dg – diagonal branch, Mg – marginal branch, Cx – circumflex branch, PDA – posterior descending coronary artery, RCA – right coronary artery, CABG – coronary artery bypass grafting, PCI – percutaneous coronary intervention, AVR/CABG – aortic valve replacement through sternotomy plus coronary artery bypass grafting, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
Data describing the performed procedures are presented in Table II. CPB, aortic cross-clamp, and operation times were statistically significantly longer in the AVR/CABG group. When a lesion was present in LAD, the surgical revascularization (LIMA to LAD bypass grafting) was performed more frequently than PCI of the LAD (p = 0.004). There was no difference between groups regarding multivessel bypass grafting or multivessel PCI performed. In the hybrid group, 65.1% of patients received DES, 33.7% received BMS, and in 1 patient balloon angioplasty without stent placement was performed.
Table II
Procedural characteristics
[i] Continuous variables are described by median [1st and 3rd quartile], categorical variables are shown as a percentage, n – number of patients, CPB – cardiopulmonary bypass, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, LAD – left anterior descending coronary artery, Dg – diagonal branch, Mg – marginal branch, Cx – circumflex branch, PDjA – posterior descending coronary artery, RCA – right coronary artery, BMS – bare metal stent, DES – drug eluting stent, CABG – coronary artery bypass grafting, AVR/CABG – aortic valve replacement through sternotomy plus coronary artery bypass grafting, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
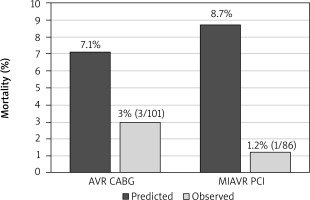
Mortality data are presented in Figure 1. Predicted hospital mortality for the entire RT-AVR/PCI group was around 8.7%, while in the AVR/CABG group it was 7.1%. Hospital mortality was lower in the RT-AVR/PCI group (1.2% vs. 3.0%); however, the difference was not statistically significant (p = 0.237). Data describing postoperative complications are presented in Table III. Both hospital stay and ICU stay were statistically significantly shorter in the RT-AVR/PCI group (p < 0.001).
Table III
Complications
[i] Continuous variables are described by median [1st and 3rd quartile], categorical variables are shown as a percentage, n – number of patients, ICU – intensive care unit, LOS – low output syndrome, AVR/CABG – aortic valve replacement through sternotomy plus coronary artery bypass grafting, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
Figure 1
Mortality. There was no difference between groups regardsing predicted mortality. Observed hospital mortality was lower in the hybrid group, however the difference was not statistically significant (1.2% vs. 3.0%, p = 0.237)
AVR CABG – aortic valve replacement through sternotomy plus coronary artery bypass grafting, MIAVR PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
The overall complication rates were significantly lower in the RT-AVR/PCI hybrid group compared with the control AVR/CABG group (18.6% vs. 33.7%, p = 0.020). The difference in complication rate was predominantly caused by a reduced rate of deep chest wound infections (4.9% vs. 0.0%, p = 0.036), less superficial chest wound infections (8.9% vs. 1.2%, p = 0.019), as well as by reduced postoperative blood loss (p < 0.001) and blood product requirements (p = 0.030).
In our material there were 3 indications for performing the hybrid procedure consisting of RT-AVR and PCI. The main indication was for patients with aortic valve disease and coronary artery disease who presented ACS necessitating urgent PCI. The second indication was the intention to perform the less invasive procedure instead of conventional AVR/CABG through the sternotomy approach. The third was the necessity to replace CABG with PCI due to the lack of good quality vascular grafts for CABG. These data are presented in Table IV.
Table IV
Indications for performing hybrid procedure
| Characteristics | Hybrid group (RT-AVR/PCI) n = 86 |
|---|---|
| PCI instead of CABG due to poor graft quality, % (n) | 4.6 (4) |
| PCI due to ACS, % (n) | 77.9 (67) |
| Less invasive instead of invasive procedure, % (n) | 17.4 (15) |
[i] Variables are shown as a percentage, n – number of patients, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention, PCI – percutaneous coronary intervention, CABG – coronary artery bypass grafting, ACS – acute coronary syndrome.
There were 47 elderly (above 75 years old) patients in our study. Data regarding elderly patients are presented in Table V. For this group of patients the predicted hospital mortality was 11.3 ±7.0% in the AVR/CABG group and 8.7 ±3.8% in the RT-AVR/PCI hybrid group (p = 0.206). There was no hospital mortality present in the RT-AVR/PCI group among elderly patients. Three patients above 75 years old from the AVR/CABG group died (13.0%, p = 0.109). Both hospital stay and ICU stay for elderly patients were statistically significantly shorter in the RT-AVR/PCI group (p < 0.001). Postoperative complications occurred in 78.3% of elderly patients from the AVR/CABG group and in 20.8% from the RT-AVR/PCI group (p < 0.001).
Table V
Elderly patients (> 75 years old, n = 47)
[i] Continuous variables are described by median [1st and 3rd quartile], categorical variables are shown as a percentage, n – number of patients, ICU – intensive care unit, LOS – low output syndrome, AVR/CABG – aortic valve replacement through sternotomy plus coronary artery bypass grafting, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
In the RT-AVR/PCI subgroup of elderly patients we noticed a statistically significant diminished rate of prolonged ventilation time, low output syndrome (LOS) and deep chest wound infections, as well a reduced volume of postoperative blood drainage and blood transfused in the postoperative period. Postoperative myocardial infarction was not present in the RT-AVR/PCI group and was 17.4% in the AVR/CABG group (p = 0.050). There was no difference between groups regarding postoperative renal failure or stroke rates among elderly patients.
Regarding the sequence of procedures, we can report 2 strategies for the RT-AVR/PCI hybrid group. The first strategy was a combined procedure in which RT-AVR was performed in the operating room, and immediately following surgery the patient was transferred to the catheterization laboratory and PCI was performed (group A). The second strategy was a staged procedure, with PCI performed first and RT-AVR performed as a separate component within 90 days of PCI (group B). These data are presented in Table VI.
Table VI
Non-staged (one-stage) and staged (two-stage) hybrid procedures (RT-AVR/PCI)
[i] Continuous variables are described by median [1st and 3rd quartile], categorical variables are shown as a percentage, n – number of patients, ICU – intensive care unit, AVR – aortic valve replacement through sternotomy, CABG – coronary artery bypass grafting, UA – unstable angina, NSTEMI – non-ST elevation myocardial infarction, STEMI – ST elevation myocardial infarction, NYHA – New York Heart Association, CCS – Canadian Cardiovascular Society, EF – ejection fraction, RT-AVR – minimal invasive aortic valve replacement through right minithoracotomy, PCI – percutaneous coronary intervention, BMS – bare metal stent, DES – drug eluting stent, RT-AVR/PCI – minimal invasive aortic valve replacement through right minithoracotomy plus percutaneous coronary intervention.
There were 21 patients in the one-stage subgroup and 65 patients in the two-stage subgroup. The percentage of elderly patients (above 75 years) was higher in the one-stage subgroup, 47.6% vs. 21.5%, respectively (p = 0.021). The number of patients who presented UA, NSTEMI, or STEMI before the procedure was higher in the two-stage subgroup (p = 0.003, p = 0.005, and p = 0.005 respectively). This was reflected by the presence of coronary symptoms (CCS class III and IV) and reduced left ventricle ejection fraction (EF) in the two-stage subgroup before PCI, which was the first part of the hybrid procedure. In the one-stage subgroup there were no patients with preoperative NSTEMI or STEMI, and 2 patients showed symptoms of UA.
In our material, the primary indication for the two-stage procedure with PCI being performed weeks or months before RT-AVR was ACS. ACS was present in 100% of patients before PCI in the two-stage subgroup and only in 9.5% of patients in the one-stage subgroup (p < 0.001). For the one-stage subgroup the main indication for the one-stage procedure, observed in 71.4% of patients, was the intention to perform the elective less invasive procedure of RT-AVR/PCI instead of conventional AVR/CABG through median sternotomy. A lack of good quality vascular grafts for CABG was the second indication for the one-stage hybrid procedure, observed in 19.1% of patients from this subgroup.
No patients from group A received dual antiplatelet therapy before the surgical part of the hybrid procedure. From group B, 32.3% of patients were on single (aspirin only) antiplatelet therapy until RT-AVR (clopidogrel was stopped 7 days before surgery), 29.2% of patients were on dual antiplatelet therapy until RT-AVR, and in 38.5% of patients both aspirin and clopidogrel were stopped 7 days before RT-AVR. Postoperative myocardial infarction was observed in one patient (4.8%) from the one-stage subgroup and in none from the two-stage group (p = 0.077). Postoperative renal failure occurred in 1 (4.8%) patient from the one-stage subgroup and in 7 (10.8%) from the two-stage group (p = 0.410). There was also no difference between subgroups regarding reoperation for bleeding or requirement for blood transfusion.
Multivariable logistic regression analysis suggests that UA, representing here ACS, was a factor associated with the decision to perform RT-AVR/PCI in a staged fashion for patients with aortic valve disease and unstable coronary artery disease (p = 0.009). These data are presented in Table VII. NSTEMI or STEMI were not observed in the one-stage subgroup and therefore were not included in the analysis.
Discussion
The hybrid approach for patients with aortic valve disease and coronary artery disease is a concept that is based on the combination of minimally invasive aortic valve surgery and percutaneous coronary intervention. The aim of this approach is to reduce surgical invasiveness with the implementation of less invasive cardiac surgery procedures instead of the conventional full sternotomy technique [10–12]. It has already been suggested that avoiding conventional CABG and replacing it with modern percutaneous techniques offers the opportunity to expand the indications for minimally invasive valve surgery to patients with concomitant coronary disease [13–15]. Replacing full median sternotomy with right minithoracotomy for aortic valve surgery should result in reduction of surgical perioperative trauma, preserved stability of the thorax, and improved respiratory function in the early postoperative period [16]. There are still problematic issues connected with the hybrid treatment of cardiac disease; these are the sequence of both components, the duration of the interval between procedures, and antiplatelet therapy [14, 15].
In our material, conventional AVR/CABG through median sternotomy was avoided in the case of aortic valve disease and unstable coronary artery disease. All patients with aortic valve disease and unstable coronary disease were treated with the RT-AVR/PCI hybrid approach.
Coronary artery disease also influenced the decision of the surgical strategy chosen. When coronary lesions in LAD were found on the preoperative coronary angiogram, patients were preferentially qualified for AVR and LIMA to LAD grafting, which was, in our heart team’s opinion, a better option than PCI of the LAD.
There is evidence in the literature that minimally invasive cardiac surgery reduces morbidity and mortality compared with conventional cardiac surgery through median sternotomy; however, some investigators have failed to demonstrate a reduction in mortality when minimally invasive techniques have been compared with heart valve surgery performed through the median sternotomy approach [16–20].
Predicted hospital mortality was high in our series – around 7.9% average for both groups. The reason for this may be that the EuroSCORE II scale we used could somehow overestimate mortality for patients with valvular heart disease [6]. In our patients we observed low hospital mortality in the RT-AVR/PCI hybrid group of 1.2%; however, it was not statistically significantly lower than hospital mortality for the control group operated on through full median sternotomy, which was 3.0% (p = 0.237).
The overall complication rates were significantly reduced in the RT-AVR/PCI hybrid group. As we expected, diminished surgical invasiveness with the RT-AVR/PCI hybrid technique resulted in reduction of blood transfusion requirements compared with AVR/CABG patients. The surgically more demanding minimally invasive approach to the aortic valve did not result in an increased number of reoperations for bleeding.
There were 3 indications for performing the hybrid procedure of RT-AVR/PCI.
The reason behind the first indication for RT-AVR/PCI is that when ACS is present, PCI allows coronary perfusion to be stabilized, and as a next step RT-AVR can be performed without increased risk, during the same hospital stay or even a few months later, as in our series. This scenario allows urgent concomitant coronary and valve surgery to be avoided in patients with unstable coronary disease, which can result in high perioperative morbidity and mortality [2].
The intention to perform less invasive RT-AVR/PCI instead of AVR/CABG is because RT-AVR/PCI is a lower-risk procedure compared with AVR/CABG [14, 15]. This procedure is even more reasonable for elderly, higher-risk patients with comorbidities, as our results confirm. Finally, the reason for the third indication is the necessity to replace CABG with PCI due to a lack of good quality vascular grafts for CABG, because it is reported in literature that the hybrid approach for patients with aortic valve disease and coronary artery disease can be applied in the case of poor-quality vascular grafts for CABG surgery [14]. On the other hand, even if saphenous vein grafts (SVG) are available, DES seems to be the better option, because it is known that SVG can have a failure rate as high as 30% during the first year after surgery [13].
As mentioned above, elderly patients and patients with comorbidities are a higher-risk population for cardiac surgery, whose postoperative course can be complicated and ICU stay can be longer [21, 22]. Analysing the selected group of elderly patients, we noticed a reduction of hospital mortality in the RT-AVR/PCI group; however, it was not statistically significant. These observations have encouraged us to apply the RT-AVR/PCI hybrid technique more frequently in the high-risk and elderly population of patients.
In this paper we report our experience with 2 strategies for RT-AVR/PCI hybrid surgery. The first strategy was a combined procedure in which RT-AVR was performed in the operating room and immediately followed by PCI in the catheterization laboratory. This strategy was called the one-stage procedure [23]. The second strategy, called the two-stage procedure, was to perform PCI first weeks before RT-AVR [2]. More elderly patients were operated on using the one-stage procedure. The intention to offer this group of higher-risk patients the less invasive treatment method instead of conventional AVR/CABG through median sternotomy was the main indication for one-stage hybrid surgery. The primary indication for the two-stage hybrid procedure in our study was aortic valve disease and unstable coronary artery disease. Multivariate analysis suggests that UA, representing here ACS, was a factor influencing the decision to perform the two-stage hybrid procedure. NSTEMI or STEMI as independent variables were not observed in the one-stage subgroup and therefore were not included in the analysis. A lack of good quality vascular grafts for CABG was the second indication for performing the one-stage hybrid procedure in our patients.
Antiplatelet therapy after PCI is another important aspect of the studied hybrid procedures. For the one-stage procedure, antiplatelet therapy was not a factor that could potentially increase the risk of postoperative bleeding because the surgical part (RT-AVR) of the hybrid procedure was performed first and immediately followed by PCI. All patients from the one-stage hybrid subgroup received loading doses of aspirin and clopidogrel after RT-AVR. In the two-stage hybrid subgroup, all patients received antiplatelet therapy before RT-AVR. Around one-third of patients from the two-stage subgroup were on single antiplatelet therapy with aspirin until RT-AVR because clopidogrel had been stopped 7 days before surgery. One-third of patients were on dual antiplatelet therapy until RT-AVR, and for slightly more than one-third of patients, both aspirin and clopidogrel were stopped 7 days before RT-AVR. In analysing these 3 scenarios we noticed that being on dual antiplatelet therapy until surgery did not increase the risk of postoperative bleeding. On the other hand, stopping dual antiplatelet therapy before surgery did not increase the incidence of postoperative myocardial infarction in our study.
We performed RT-AVR when patients were on clopidogrel and did not observe any increased bleeding rate and blood product requirement, so in our opinion the most important issue is the correct surgical technique.
The sequence of procedures is also a matter of debate [14]. First PCI then RT-AVR is one option, and this was a sequence we used in the two-stage hybrid subgroup, performed due to ACS. The advantage of this strategy is that patients undergo aortic valve surgery without having severe stenosis of the coronary arteries. The disadvantage is the potentially increased risk of postoperative bleeding due to antiplatelet therapy after PCI.
The second option used in the one-stage subgroup is to perform RT-AVR first, followed by PCI. The advantage of this strategy is the reduced risk of increased perioperative bleeding [24]. However, in our patients, we did not notice any increased use of blood products when patients were operated on, even when on dual antiplatelet therapy. The disadvantage is the potentially increased risk of postoperative myocardial infarction because the surgery is being performed with severe stenosis of the coronary arteries [22].
In our study we were able to demonstrate that in a group of non-selected patients with coronary artery disease and aortic valve disease, a hybrid approach based on minimally invasive aortic valve replacement through right minithoracotomy and percutaneous coronary intervention gives superior results in comparison with conventional aortic valve replacement and coronary artery bypass grafting through median sternotomy. The study has shown that the hybrid approach for treating patients with aortic valve disease and coronary artery disease can be performed without increased risk of hospital mortality and perioperative morbidity even in elderly patients.
Additional information could be obtained after comparing the described surgical strategy with the new percutaneous technique of aortic valve implantation [25].
There are some limitations of our study. It was a limited, single-center, non-randomized study. The number of patients was not large, so our results are exclusive to minimally invasive aortic surgery through right minithoracotomy and cannot be extrapolated to other techniques. The profile of studied groups was not strictly homogenous – the hybrid group consisted of 2 subgroups of patients and in the one-stage subgroup, elective patients were included. The two-stage subgroup consisted of patients upon whom urgent PCI was performed as a first part of the hybrid procedure, which was followed by electively scheduled RT-AVR within 90 days of PCI. Moreover, there was no strict period in the study protocol, which separated RT-AVR from PCI in the two-stage subgroup of patients. For most patients this period was 1–2 months, and there were no patients with an interval between the two procedures longer than 90 days.
The RT-AVR/PCI hybrid technique also has some limitations. From the surgical side these are limitations typical for minimally invasive aortic valve surgery such as the presence of a learning curve. Exposing the aortic valve can be more difficult, which can cause some discomfort for a surgeon, especially when first undertaking the minimally invasive aortic valve surgery program. Finally, aortic cross-clamp time and operative time are longer compared with isolated AVR through conventional median sternotomy.
Although in many situations cardiac surgery is the treatment of choice, nowadays we may also use the technique of interventional cardiology.
According to the 2021 ESC/EACTS Guidelines for the management of valvular heart disease, patients with symptomatic severe aortic stenosis may be qualified for the TAVI procedure or classic cardiac surgery [26]. TAVI is a preferable method in patients above 75 years old or with high perioperative risk with STS PROM/EUROSCORE II > 8%. Reports show that 40–75% of TAVI patients are suffering from coronary artery disease (CAD). The coexistence of CAD is a predictor of mortality being independent of other risk factors, which is consistent with the observations of patients undergoing classic surgery. D’Ascenzo et al. suggested that the risk of death after TAVI may closely convey the complexity of CAD. The 1-year mortality rate seems to be higher in patients with a syntax score > 22 and lower in those with an syntax score < 8 [27].






