Introduction
Surgical aortic valve replacement (SAVR) in patients affected by severe aortic valve disease, i.e., stenosis or steno-insufficiency, is still performed today, with a very low operative risk for mortality and stroke, 1.9% and 1.2%, as reported by the STS Adult Cardiac Surgery Database in 2021 [1]. However, in the last decade, the clinical profile of patients referred for aortic valve surgery has tended to worsen, with an increase in patients over 75 years of age, with more associated pathologies, i.e., chronic obstructive pulmonary disease, renal dysfunction, peripheral arterial vascular disease, obesity, and in the presence of a greater incidence of worse general conditions due to frailty or multi-organ pathology, with undoubtedly an increase in the risk calculated by the surgical scores currently in use, such as American STS and European scores. On the other hand, the percutaneous treatment of the aortic valve with the implantation of a biological prosthesis, called trans-catheter aortic valve replacement (TAVR), has undergone notable progress with a significant reduction in the incidence of major complications such as paravalvular leaks and peripheral vascular lesions, which have allowed the extension of the indications to a percutaneous procedure, initially reserved for high-risk or inoperable patients, even in the presence of intermediate and low risk, over 75 years of age. For these reasons, the latest 2021 European Guidelines, for patients over the age of 75 or STS-Prom score/EuroScore-2 ≥ 8%, place the TAVR procedure in Class I indications [2]. However, the hemodynamic profile of the latest generation biological aortic valve prostheses has significantly improved, and the possibility of alternatively using non-stented biological prostheses (so-called suture-less prostheses) allows surgical results to be obtained that are still very satisfactory today. In fact, the advantage of the valve replacement that still remains undisputed today is that of being able to accurately and openly remove para-annular calcifications (unlike the TAVR procedure), which are responsible for the postoperative and follow-up risk of stroke. The Perceval suture-less bio-prosthesis (Corcym; previously produced by Sorin Group and LivaNova) has been developed to combine the advantage of rapid deployment with that of the traditional surgical approach [3–5]. It is a valve made of bovine pericardium attached to a self-expanding nitinol cage. This prosthesis was introduced in 2007 and has shown stable hemodynamic performance with very satisfactory safety, postoperative gradients, and early and mid-term follow-up results. Several studies have shown that the Perceval implant reduces cardiopulmonary and aortic cross-clamp times in comparison with sutured biological prostheses, thus enhancing a more favorable recovery, especially in older patients, with chronic respiratory pathologies and associated comorbidities. Moreover, the Perceval may enable lower peak and mean pressure gradients than conventional biological prostheses, higher effective orifice area and therefore lower risk of patient-prosthesis mismatch. In comparison with sutured prostheses, disadvantages of the Perceval valve are related to the selective implantation criteria, due to which it is not possible in all patients, i.e., in relation to the annular and the sino-tubular junction diameters, the incorrect positioning, which can cause para-valvular leaks, and the excessive stretching by the oversizing on the native annulus, which can more easily cause postoperative AV block and, consequently, the increase of the pacemaker implantation rates [3, 4].
Aim
Starting from the hypothesis on the possible advantages that the Perceval sutureless biological prosthesis can provide in SAVR surgery, we began in the year 2022 a retrospective observational monocentric study entitled “Hemodynamic performance and medium-term results of patients undergoing aortic valve replacement with Perceval sutureless bioprosthesis compared with implantation of the third-generation St. Jude Trifecta biological aortic valve prosthesis – Per-fecta Study”, with the aim to evaluate, in the first analysis of the proposed study, the immediate results, i.e., operative mortality, incidence of major complications, hemodynamic performance at discharge of the Perceval implant in comparison with the sutured biological prosthesis of the latest generation St. Jude Trifecta (St. Jude Medical, Abbot Vascular, previously St. Jude Medical, Inc., St. Paul MN, USA).
Material and methods
From December 2014 to June 2023, at the Cardiac Surgery Division of the Tor Vergata University of Rome – Tor Vergata University Polyclinic, 281 patients (152 males, 54%, 68 females, 46%) underwent SAVR as an isolated intervention (n = 181, 64.4%) in association with CABG (n = 100, 35.6%). A St. Jude Trifecta sutured bioprosthesis was implanted in 220 (78.3%) patients and a Perceval sutureless bioprosthesis in 61 (21.7%).
The Perfecta study was approved by the Institutional Review Board – Ethics Committee of Tor Vergata Polyclinic (No. 107/22, date of approval 14 June 2022). The two groups of patients mentioned above were the subject of our retrospective study.
Clinical variables
For both groups of patients, gender, age, height, and weight were evaluated; obesity was defined in presence of a body mass index equal to or greater than 30. Clinical conditions were assessed at admission, i.e., the risk score of the patient calculated by the EuroSCORE II evaluation system (European System for Cardiac Operative Risk Evaluation), NYHA class, the presence of cardiovascular risk factors, i.e., smoking, diabetes mellitus, dyslipidemia, hypertension, the presence and extent of concomitant coronary artery disease, left main disease, and the indication for surgery, i.e., elective or urgent.
Concomitant pathologies
The following pathologies were considered: obesity, expressed as body mass index and defined as a value > 30 kg/m; chronic obstructive pulmonary disease, expressed as forced expiratory volume in 1 second < 75% of the normal value, or requiring bronchodilators. Arterial peripheral vascular disease was considered to be significant in presence of claudication with autonomous walking < 200 m and low peripheral artery pulsatility; carotid artery disease in presence of stenosis > 50% detected in ultrasound echo-color Doppler examination. Chronic renal dysfunction was defined as moderate in presence of creatinine clearance 50–80 ml/min, severe with creatinine clearance < 50 ml/min.
Echocardiographic parameters
Trans-thoracic echocardiography was performed in all patients preoperatively and at discharge. Standard aortic valve and prosthetic valve measurements were obtained in accordance with the American Society of Echocardiography criteria [6, 7]. Peak and mean trans-valvular and trans-prosthetic valve gradients, effective orifice area and indexed effective orifice area (iEOA), left ventricular ejection fraction, end-systolic and end-diastolic diameters, interventricular septum and posterior wall thicknesses, systolic pulmonary arterial pressure, and right ventricular function were assessed. The degree and the severity of associated aortic valve regurgitation were also assessed [7]. Patient-prosthesis mismatch was defined as mild-to-moderate in presence of iEOA > 0.80 cm2/m2 and ≤ 0.85 cm2/m2, moderate in presence of iEOA > 0.65 cm2/m2 and ≤ 0.80 cm2/m2, severe in presence of iEOA < 0.65 cm2/m2 [8].
Surgical techniques
A complete sternotomy or a mini-sternotomy approach with a “J” incision was performed in all patients. Once cardiopulmonary bypass was started, after cross-clamping the ascending aorta and performing the cardiac arrest using warm blood cardioplegia [9], St. Thomas cold crystalloid cardioplegia [9], or Custodiol HTK solution, the proximal aorta was opened with a transverse aortotomy, 1.0–1.5 cm distally to the origin of the right coronary artery and extended circumferentially for the Trifecta implantation, 3.0–3.5 cm higher from the aortic annulus for the Perceval implant. Excision of the cusps was started with scissors according to the techniques currently described in the literature, following an accurate and as complete as possible decalcification of the native aortic annulus. St. Jude Trifecta implantation was performed using 10–16 double-needled 2–0 stiches with Teflon pledgets, in accordance with the size of the prosthesis at the sub-annular position. The Perceval prosthesis, after measuring the size to be implanted, was released onto the native annulus and fixed in place by inflating the balloon to 3–4 atmospheres. After placement of prostheses, the aortotomy was closed with a 4–0 polypropylene double continuous suture. Concomitant coronary artery bypass grafting was performed using the left internal thoracic artery to graft the left anterior descending artery and saphenous vein grafts for the revascularization of the right coronary artery and circumflex artery territories. Trans-oesophageal echocardiography was performed intraoperatively and at weaning from cardiopulmonary bypass in all cases.
The size of the implanted St. Jude Trifecta prostheses was 19 mm in 61 (27.7%) patients, 21 mm in 88 (40%), 23 mm in 52 (23.6%), and 25 mm in 19 (8.6%). Sizes of the implanted Perceval were S (small) in 16 (26.2%) patients, M (medium) in 34 (55.7%), L (large) in 8 (13.1%), and XL in 3 (5.0%).
Definitions and data analysis
The current study considered all consecutive patients undergoing SAVR as an isolated procedure or in association with CABG. Other surgical operations performed in association with repair or replacement of other heart valves, or in association with ascending aorta repair, were not included. All patients gave their informed surgical consent.
Operative or in-hospital mortality included deaths occurring in the hospital or within 30 days after the SAVR operation. The main cardiac and non-cardiac postoperative complications analyzed were perioperative myocardial infarction, defined as an increase of creatine-kinase muscle-brain (CK-MB) enzyme greater than 10% of the total CK enzyme, and the onset of ECG anomalies associated with an increase of serum troponin I > 20 ng/ml. Low cardiac output syndrome was defined in the presence of a cardiac index value lower than 2.0 l/min/m2 requiring inotropic drugs for a period greater than 48 hours or when intra-aortic balloon pump insertion was needed. Respiratory failure was defined as an episode of primary respiratory insufficiency requiring mechanical support for a period greater than 48 hours, tracheal re-intubation, intermittent application of non-invasive positive-pressure ventilation, or percutaneous tracheostomy.
Stroke was defined as a neurological complication due to a focal cerebral lesion; acute renal impairment was defined as a two-fold increase of preoperative serum creatinine level or oliguria necessitating mechanical hemofiltration.
Statistical analysis
Statistical analysis was performed using Stat View 4.5 (SAS Institute Inc., Abacus Concepts, Berkeley, CA). Contingency tables’ raw data with the use of chi-, G-squared, and Fisher’s exact tests for categorical variables and the unpaired Student’s t-test for continuous variables were calculated to perform the comparisons of the two groups of patients receiving Perceval or Trifecta prostheses. A total of 31 variables were analyzed among the demographic, clinical and echocardiographic preoperative variables mentioned above. Intraoperative analyzed variables included the type of cardioplegia administered, cardiopulmonary bypass and aortic cross-clamp times. Univariate analysis of preoperative and intraoperative variables considered as potential risk factors of operative mortality and postoperative major complications was performed; all the variables that reached a p-value < 0.1 were included in the multivariate logistic regression analysis.
Operative mortality and major complications were also evaluated as the only variable, in order to obtain greater statistical power of the study. All continuous variables were expressed as mean ± 1 standard deviation. P-values < 0.05 were considered statistically significant.
Results
Clinical features and associated pathologies
Overall, the preoperative clinical demographic variables were similar in the two groups of patients. As compared with the St. Jude Trifecta group, the Perceval group at admission had older age and higher NYHA class (p < 0.01, for the comparisons) (Table I). The higher age of the Perceval implant was due to the fact that in the first period of activity we chose the over 75 age criterion recommended by the guidelines for the implantation of a sutureless prosthesis; subsequently, we also implanted this prosthesis for age under 75 years. More patients in the Trifecta group required urgent surgery due to a more serious clinical presentation related to the severity of the associated coronary artery disease (p < 0.01) (Table I).
Table I
Clinical preoperative characteristics of patients undergoing aortic valve replacement with Perceval sutureless or St. Jude Trifecta bioprostheses
Echocardiographic variables
The Trifecta group had a greater degree of concentric hypertrophy of the left ventricle, while the Perceval group had a greater incidence of significant aortic valve regurgitation associated with stenosis, with a consequent increase in the mean end-systolic diameter value. The measured maximum peak and mean trans-valvular gradients highlighted a similar degree of severity of aortic stenosis (Table II).
Table II
Preoperative echocardiographic variables
Intraoperative data
The Perceval implant significantly reduced, as expected, extracorporeal circulation and aortic cross-clamping times, i.e., by over 30 and 20 minutes, respectively, both in isolated valve replacement and combined with CABG (p < 0.001, for all comparisons) (Table III).
Table III
Intraoperative data
Perioperative results
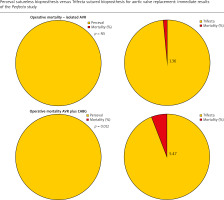
Operative mortality was 2.13% in the whole population (6/281): absent in the Perceval group, 2.7% in the Trifecta group (Table IV). Operative mortality was significantly higher in patients undergoing SAVR plus CABG with the Trifecta prosthesis compared to the Perceval group, i.e., 5.47% vs. 0% (p < 0.05) (Figure 1). The incidence of total postoperative major complications analyzed including in-hospital deaths was significantly higher in the Trifecta group. This higher incidence resulted primarily from the higher incidence of postoperative low cardiac output syndrome observed in the Trifecta group (Table IV). Independent predictors of operative mortality and major postoperative complications were the presence of concomitant severe multi-vessel and/or left main coronary artery disease (p < 0.05; HR = 4.6) and chronic pulmonary disease (p < 0.01; HR = 5.6) (Table V). Longer times of extracorporeal circulation and aortic cross-clamp were found to be risk factors for mortality and postoperative complications only in the univariate analysis (Table V); type of biological prosthesis, i.e., Trifecta vs Perceval, was not found to be a risk factor in either analysis.
Table IV
Perioperative outcomes and postoperative complications
Table V
Risk factors and independent predictors of operative mortality and postoperative complications (univariate and logistic regression analyses)
Figure 1
Comparison of operative mortality for isolated and CABG-associated aortic valve replacements with Perceval sutureless versus St. Jude Trifecta biological prostheses
Need for permanent pacemaker implantation was similar for both types of biological prostheses, i.e., 4.5% vs. 4.9%, as well as the other measured outcomes (Table IV).
Echocardiographic variables at discharge
Both types of prostheses were confirmed to be effective for the treatment of aortic valve disease, offering a substantially similar and satisfactory hemodynamic performance in comparison in terms of trans-prosthetic peak and mean gradient values, and left ventricular function.
In patients undergoing Perceval implantation, better global cardiac hemodynamic performance was also observed, demonstrated by a higher mean value of tricuspid annular plane systolic excursion (TAPSE), and by a lower incidence, reduced by about a half, of the moderate degree of patient-prosthesis mismatch (Table VI). No para-valvular leaks were observed.
Table VI
Postoperative echocardiographic variables
| Variables | Perceval (n = 61) | Trifecta (n = 220) | P-value |
|---|---|---|---|
| LV ejection fraction % (mean ± SD) | 58.7 ±4.9 | 56.9 ±6.8 | 0.10 |
| LV end-diastolic diameter [mm] (mean ± SD) | 52.4 ±6.4 | 47.4 ±6.4 | < 0.0001 |
| LV end-systolic diameter [mm] (mean ± SD) | 35.6 ±6.9 | 33.0 ±6.6 | 0.05 |
| LV end-diastolic volume [ml] (mean ± SD) | 91 ±11 | 84 ±24 | 0.24 |
| LV end-systolic volume [ml] ((mean ± SD) | 45 ±10 | 38 ±14 | 0.04 |
| LV septum thickness [mm] (mean ± SD) | 13.1 ±1.5 | 12.7 ±1.6 | 0.22 |
| Posterior wall thickness [mm] (mean ± SD) | 12.2 ±1.1 | 11.8 ±1.4 | 0.12 |
| Systolic pulmonary pressure [mm Hg] (mean ± SD) | 30.5 ±6.4 | 28.9 ±5.9 | 0.09 |
| TAPSE of the right ventricle [mm] (mean ± SD) | 26.0 ±1.8 | 16.9 ±4.1 | < 0.0001 |
| Trans-prosthetic aortic valve peak gradient [mm Hg] (mean ± SD) | 22.6 ±7.9* | 21.6 ±7.3┼ | 0.41 |
| Trans-prosthetic aortic valve mean gradient [mm Hg] (mean ± SD) | 12.6 ±4.8* | 11.6 ±4.3┼ | 0.15 |
| Patient-prosthesis mismatch, n (%): | |||
| Mild-to-moderate | 27 (44.3) | 44 (20) | |
| Moderate | 5 (8.2) | 38 (17.3) | |
| Severe | 0 | 1 (0.5) | 0.0004 |
| Para-valvular leak, n (%) | 0 | 0 | – |
Discussion
The preliminary data on the immediate postoperative outcomes of the Perfecta study confirm that both types of bioprostheses appear to be equally effective in the treatment of severe aortic valve disease with a surgical approach, with a very limited operative risk, i.e., below 3%, despite the average age of the patients being over 75 years, several associated comorbidities being present, and over a third of the patients having severe concomitant coronary disease requiring CABG.
The St. Jude Trifecta prosthesis is a tri-leaflet stented pericardial biological prosthetic valve designed for aortic valve replacement. The bovine pericardial sheet is mounted outside the stent frame, which allows for an almost circular cross-section during systole. Several studies have shown a favorable hemodynamic profile, i.e., low peak and mean trans-prosthetic gradients, excellent effective orifice area, and low incidence of patient-prosthesis mismatch, including in patients with a small aortic annulus [10–19]. Moreover, the excellent fluid dynamic characteristics of the Trifecta aortic prosthesis have also been favorably compared with those reported for stentless biological valves [20–23].
Ideally, this prosthesis designed in this way would have the aim of guaranteeing three (“Tri-fecta”) essential advantages: quick and easy implantation, excellent hemodynamic profile, and high freedom from deterioration.
In fact, in comparison with the Perceval prosthesis, which is also designed to gain a greater effective valve orifice and therefore reduce the risk of high trans-prosthetic gradients and postoperative mismatch, in our study we also observed that the Trifecta allows one to obtain trans-prosthetic valve gradients at discharge that are very favorable and comparable with those generated by the Perceval prosthesis [24–26].
The very satisfactory hemodynamic profile of the Trifecta in comparison with the Perceval prosthesis is further demonstrated by the fact that the smaller sizes (19 and 21 mm) of the Trifecta were implanted in almost two thirds of patients (67.7%), while the small size (S) of the Perceval valves (which corresponds approximately to size 19–21 mm of sutured biological prostheses) was implanted in only 26% of cases.
In fact, as expected, the greater use of sizes M (which corresponds approximately to size 21–23 mm of sutured biological prostheses) and L in the Perceval group of patients has allowed the incidence of the moderate degree of patient-prosthesis mismatch to be reduced.
Obviously, the degree of mismatch, more than in the immediate post-operatory period, will have an influence on the hemodynamic performance in the medium and long term, and, above all, on the freedom from structural valve deterioration and on the event-free survival, which will be the subject of our further investigations.
Regarding the observed in-hospital outcomes, we focus on some clinical and surgical aspects. The Perceval implant was associated, as widely reported in the literature [4, 5, 25, 27–29], with reduced cardiopulmonary bypass and aortic clamping times. In particular, we observed an overall reduction of more than 30 and 20 minutes, respectively, in both the isolated and combined procedures. In the univariate statistical analysis, the reduction in surgical times translated into a significant reduction in postoperative complications after Perceval implantation, especially in the risk of low output cardiac syndrome. Undoubtedly, part of the risk of low output syndrome could derive from the fact that the patients in the Trifecta group presented themselves for surgery with a higher EuroScore-2, albeit without statistical significance, and more frequently in urgently conditions (p < 0.01), but certainly a reduction in aortic clamping of more than 20 minutes, and consequently cardiac arrest time, likely may have had a protective effect in the development of this complication, thus allowing a more rapid recovery of the myocardial function. The same can be said regarding global cardiac systolic function, i.e., right ventricular function, which was shown to be better after Perceval implantation. These advantages related to the reduced surgical implantation time may be greater especially in elderly patients suffering from pre-operative comorbidities, such as the presence of chronic obstructive pulmonary disease, and serious associated pathologies such as coronary artery disease. In the multivariate analysis, we observed that the independent predictive factors of major postoperative complications were precisely the two factors indicated above. The mortality rate observed in our series, i.e., absent in the Perceval group and 2.7% in the Trifecta group, is comparable to that previously reported in the Perceval and in the Trifecta studies, both monocentric and multicentric, and meta-analyses [3–5, 11–17, 25, 27–32]. Contrary to what has been reported in other studies [27, 28, 31], the incidence of pacemaker implantation and para-valvular leaks in the Perceval group was limited and substantially similar to that observed for the Trifecta valve. We cannot say with certainty the reason for these findings. Given that the implantation of the Perceval was mainly carried out by a single operator (i.e., G.R.), an expert in its positioning, this aspect could, at least in part, have contributed to the reduction in the incidence of the two complications mentioned above. Starting at the end of 2022, the manufacturer Abbot initiated a market withdrawal for the Trifecta family of valves, for which we are carrying out the evaluation of the clinical and echocardiographic results of the follow-up.
Strengths and limitations of the study: The primary interest of the study is that we compared two types of latest generation prostheses, Perceval vs. Trifecta, to evaluate their effectiveness, and we observed that with them, the immediate postoperative results of SAVR continue to be very satisfactory, despite the age and incidence of comorbidities of patients referred for surgery continuing to increase. The main limitations of the study are related to the small sample of patients who had the Perceval implanted. This may underestimate the incidence of some problems related to its implant, such as para-prosthetic leak and postoperative AV blocks. Furthermore, to fully evaluate the effectiveness, safety, and reliability of the two types of prostheses, accurate evaluation will be necessary during medium- and long-term follow-up.
Conclusions
Surgical aortic valve replacement in elderly patients with latest generation bioprostheses is associated with limited operative risk, i.e., 2% isolated mortality, 4% plus CABG. Both the Trifecta sutured and Perceval sutureless biological prostheses appear to guarantee a satisfactory hemodynamic profile at discharge. Due to the reduction of surgical time, Perceval prosthesis implantation can lead to a significant reduction in operative mortality and major complications, as a result of better control of risk factors and better preservation of myocardial contractile function.






