Introduction
Managing congenital heart defects in patients with haemophilia A, particularly in cases of severe factor VIII deficiency, presents a multidisciplinary challenge [1–3]. While some successful procedures and attempts to standardize treatment approaches, including peri-procedural recommendations for the multidisciplinary care team, have been described, an individualized approach is warranted [2, 3].
Case report
We present a peri-procedural approach resulting in the successful transcatheter closure of a large secundum atrial septal defect (ASD II) in a significantly compromised 3-year-old boy (11.8 kg).
The patient was born prematurely at 28 weeks of gestation (1300 g), with genetically determined factor VIII deficiency – severe haemophilia A, associated with less than 1% factor VIII activity. He has had multiple complications related to prematurity, including intraventricular haemorrhage (grade III), intracranial haemorrhage, hydrocephalus, ventriculo-peritoneal shunt insertion, bronchopulmonary dysplasia, retinopathy of prematurity, and psychomotor disturbances.
Due to severe haemophilia A with extremely low factor VIII levels and exceptionally unstable factor VIII, the patient had been receiving recombinant factor VIII infusions (Octocog alfa, Advate, Taakeda Pharma) three times a week. Despite this, he experienced moderate intracranial haemorrhage. Using an online population-based application (MyPKFiT), his factor VIII pharmacokinetics (PK) were measured, showing a very short half-life of factor VIII – 6.6 h. Based on this information, an individual regimen of prophylaxis was established to obtain the proper level of factor VIII. The boy started receiving factor VIII infusion every second day. Additional infusions were required before any interventions associated with increased bleeding risk.
Based on the medical documentation, due to exceedingly high procedural risk, the patient was initially deemed ineligible for both surgical and interventional treatment. Additionally, the large size of the ASD and the small rims raised concerns regarding the success of the transcatheter treatment in the young patient.
However, during rehabilitation, the parents noticed a continuous decrease in exercise tolerance and sought a second opinion.
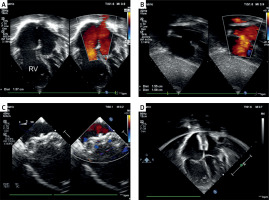
A transthoracic echocardiogram revealed a significantly enlarged right atrium and ventricle, ASD II with its largest dimension being measured at 1.58 cm, significant left-to-right shunt, (estimated Qp : Qs of 3.3 : 1), dilated right ventricle (2.9 cm measured in parasternal long axis in diastole), main pulmonary artery (25 mm) and branches, but no signs of pulmonary hypertension (peak gradient of tricuspid valve regurgitation 23 mm Hg) (Figures 1 A, B).
Figure 1
Percutaneous closure of a large secundum atrial septal defect in a patient with severe haemophilia A. A – Transthoracic echocardiography, four-chamber view: a large central secundum-type atrial septal defect with an enlarged right atrium and right ventricle (RV). B – Transthoracic echocardiography, subcostal view: a large central defect with adequate rims. C – Transoesophageal echocardiography, mid-oesophageal projection, 0 degrees illustrating proper orientation of the released Amplatzer Septal Occluder (Abbott). D – Follow-up transthoracic echocardiography, four-chamber view revealing the device well aligned with the atrial septum, distant from the atrioventricular valves, with persistent dilation of the right atrium and right ventricle
After interdisciplinary discussion, the patient was disqualified from surgical treatment due to underlying conditions and extremely high operative risk. Hence, the remaining option was an interventional attempt at closing the large ASD.
Detailed peri-procedural management to prevent bleeding was prepared in agreement with a haematology specialist (Table I). On the day of the procedure, a recombinant factor VIII infusion of 1000 units was administered, with factor VIII levels being measured at 68%, which was closely monitored in the following postoperative days.
Table I
Periprocedural protocol for maintenance of adequate levels of factor VII
| Time | Intravenous dose of recombinant factor VIII (international units [IU]) | Total number of doses per time interval | Required factor VIII activity |
|---|---|---|---|
| Before intervention: | |||
| Current treatment | Infusion 750 IU | One dose every 2nd day | Depending on the indications |
| On the day of intervention: | It is required to achieve target factor VIII activity of at least 90% on the day of the procedure.** | ||
| 1 h before the procedure: | Infusion 1000 IU (1st dose) Heparin was not ordered | Single dose | |
| 2 h after administration of the 1st dose | Infusion 250 IU | Single dose | |
| Every 2 h for the next 12 h | Infusion 250 IU | A total of 6 doses of 250 IU within 12 h | |
| Every 3 h for the next 12 h | Infusion 250 IU | A total of 4 doses of 250 IU within 12 h | |
| On the 2nd day after the procedure: | It is required to achieve target factor VIII activity minimum 60% on the 2nd and 3rd day after the procedure.** | ||
| Every 4 h for the next 24 h | Infusion 250 IU | A total of 6 doses of 250 IU within 24 h | |
| On the 3rd day after the procedure: | |||
| Every 5–6* h for the next 24 h | Infusion 250 IU | A total of 4–5* doses of 250 IU within 24 h | |
| Subsequent days after intervention: | |||
| Return to previous therapy | Infusion 750 IU | One dose every 2nd day | Depending on the indications |
Under general anaesthesia, a transoesophageal echocardiogram (TEE) was performed. It revelated a 12 × 15 mm ASD II, with 5 mm aortic rim and floppy inferior vena cava rim. The total length of the septum was measured as 38 mm. Under ultrasound guidance, standard femoral vascular access was obtained. Consistent with the recommendation that anticoagulant therapy can be safely considered with factor VIII activity maintained above > 90%, no heparin was administered (pre-procedure factor VIII levels – 68%). Using a 7 Fr Torque Vue (St. Jude Medical) delivery sheath, a 16 mm Amplatzer septal occluder (St. Jude Medical) was successfully implanted in the ASD. Subsequent TEE and fluoroscopy confirmed stable device position (Figure 1 C). Following removal of the vascular sheath, manual haemostasis was achieved without delay.
The patient was extubated in the catheterization laboratory, and further management followed haematological recommendations. Aspirin was prescribed at 3 mg/kg body weight for 6 months. No complications occurred in the days following the procedure. Post-procedural and pre-discharge transthoracic echocardiography showed a stable position of the occluder without a residual shunt (Figure 1 D).
The patient was discharged on the 7th day after implantation, with prolonged hospital stay solely due to extensive multispecialty consultations related to comorbidities, not the cardiovascular intervention. Two-year follow-up demonstrated proper device position and good ventricular function with a reduction in the right ventricular size.
Discussion
Haemophilia A, characterized by factor VIII deficiency, presents with varying severity: > 5% activity indicates a mild form, while < 1% signifies a severe form. Treating premature infants, especially extremely preterm, requires specialized approaches [1]. Modern therapies enable effective factor VIII supplementation, enhancing haemophilic patients’ life expectancy along with the general population [2]. This necessitates managing cardiovascular diseases, balancing antiplatelet and anticoagulant therapy with bleeding prevention [3]. Available guidelines for adults are often based on case reports, warranting a tailored approach for paediatrics. Minimally invasive procedures, such as percutaneous coronary intervention (PCI) instead of coronary artery bypass grafting (CABG) and percutaneous correction of cardiac defects (e.g., septal defect closure, PDA occlusion), are favoured [4–8]. However, surgical treatment is possible with proper preparation and individualized management [8–12]. Experts recommend maintaining factor VIII at 80–100 IU/dl during anticoagulant therapy tailored to the individual. For antiplatelet therapy, factor VIII should be ≥ 15–30 IU/dl for dual therapy and ≥ 1–5 IU/dl for single therapy [3]. However, limited data on antiplatelet and anticoagulant therapy in haemophilia necessitate careful risk assessment and therapy adjustment, coordinated with a multidisciplinary care team and appropriate institutional support [10].
For the presented patient, a detailed diagnostic and therapeutic process, from diagnostic imaging to multidisciplinary team discussion and the decision on eligibility for a minimally invasive procedure, enabled effective and safe percutaneous ASD occlusion. Medication management, including withholding perioperative heparin and strict post-operative monitoring, was guided by factor VIII levels. Aspirin, administered at a standard dose for 6 months, was well tolerated without bleeding. The procedure and post-operative management did not require modifications to the existing recombinant factor VIII supplementation regimen.
Conclusions
The comprehensive management strategy involved interdisciplinary collaboration and careful consideration of the patient’s complex medical history, including prematurity-related complications and haemophilia-related issues. The successful intervention demonstrates the feasibility of transcatheter procedures in high-risk patients with challenging anatomical conditions, highlighting the importance of multidisciplinary care in effective management.








