Introduction
The incidence of renal cell carcinoma (RCC) is around 3% of all cancers and has been growing for the last decades, making it one of the most rapidly increasing cancer diagnoses [1].
According to the European Association of Urology (EAU) guidelines, if technically feasible, the primary treatment for T1 RCC should be partial nephrectomy (PN). However, in smaller masses, percutaneous cryoablation (PCA) is gaining attention as a curative option with comparable oncological outcomes to PN in appropriately selected patients [2]. Moreover, PCA is a true minimally invasive procedure associated with fewer complications when compared to classic surgery and satisfactory kidney function preservation [2].
Nonetheless, like any surgical procedure, PCA is not free from complications. We aimed to assess the safety profile of PCA of renal tumours during the learning curve of the first 75 cases in our institution.
Material and methods
Informed consent was obtained from all of the patients prior to the PCA procedure. The study was approved by the Institutional Bioethics Committee of Wrocław Medical University.
All consecutive procedures performed in the University Clinical Hospital between 06.2023 and 06.2024 were analysed. PCAs were performed by an interventional radiologist highly skilled in computed tomography (CT) interventions (MG) and a urologist with vast experience in USG-guided percutaneous renal procedures (WK). The procedure methodology has been described in detail previously [3].
In our centre, PCA was not a treatment of choice for cT1 tumours. Therefore, only patients with cT1 renal tumours who were ineligible for PN surgery (54 patients) or strongly opposed to the classic operations (14 patients) were included. The patients were ineligible for PN because of anaesthesiologic reasons and/or a history of previous surgical proceedings on or in proximity to the ipsilateral kidney.
Patients were admitted with a current, reliable urine culture and received antibiotic prophylaxis accordingly. Antiplatelet and anticoagulation medications were ceased or modified according to the guidelines.
The majority of patients were sedated using intravenous remifentanil. In addition, local anaesthesia with lidocaine and bupivacaine was injected into the puncture site. In 25 cases the operations were performed solely under local anaesthesia and 1 under general anaesthesia.
In cases without prior biopsy, samples were taken during the PCA procedure.
The cryoprobes were inserted percutaneously under hybrid USG/intermittent CT guidance.
Hydrodissection with more than 100 ml of 2% contrast medium solution was performed in 9 cases.
Procedures were performed using cryoprobes from Boston Scientific (Marlborough, USA) and from IceCure (Cesa-rea, Israel).
Patients’ fear before the procedure and periprocedural pain and nausea were assessed by the patients using the 0-10 numeric rating scale (NRS).
Complications that took place during the hospital stay and in the first 4 weeks after the procedure, both during hospitalisation and after discharge, were evaluated. Major complications were defined as at least grade III in the Clavien-Dindo system. The majority of the patients were followed up by members of the surgical team. Additionally, all patients were contacted by telephone, and if any complication took place, they were asked to send the referring documents.
Finally, a Validated Tool for Assessment of Patient Satis-faction Following Surgery (SSQ-8) was used. The SSQ-8 score was determined by adding the points from 7 questions, where each question scored from 1 to 5 points (1 represented the highest level of satisfaction).
Results
Study cohort
The study included 68 patients with 75 tumours treated with PCA in our centre between 06.2023 and 06.2024. Basic patients’ demographic, clinical, and hospital characteristics are presented in Table 1. The included group was old (mean 72 years), overweight (mean BMI 29.1) and severely comorbid (average Charlson comorbidity index 5.9; ASA score 2.8). A significant proportion of the patients had a solitary kidney (28.0%) or underwent previous PN (30.7%). Nonetheless, the mean preprocedural eGFR was 77 ml/min/1.73 m2. Considering the tumours, the majority were cT1a (median 3.4 cm [interquartile range: 1.5–3.1 cm]) and had moderate complexity (mean RENAL score 7.5). The pathology was available in 65 (86.7%) of the cases and the most common result was clear-cell RCC (55.4%). Moreover, grade 1 was prevalent in clear-cell RCC and constituted 52.8% of these tumours. PCA was usually performed using the Boston Scientific system (62.7%). Finally, median preprocedural fear was moderately low (NRS 3). Patients spent a median of 19 hours (range 6–59) in the hospital. In 25 cases hospitalisation extended overnight due to severe comorbidities and the potential risk they might cause. The remaining patients who were discharged on posoperative day 1 stayed overnight in compliance with an older protocol, rather than medical necessity.
Table 1
Basic patients’ demographic, clinical, and hospital characteristics
Complications
Peri- and postoperative complications ranked by seve-rity using the Clavien-Dindo classification are presented in Table 2.
Table 2
Reported peri- and postoperative complications
Perioperative
Needle placement was associated with discomfort or low-intensity pain and nausea in 16 (21.3%) and 3 (4.0%) patients, respectively (both under local anaesthesia only and under sedation), which highlights the need for meti-culous preoperative tract analgesia. When asked postope-ratively, patients reported median pain sensation associated with the needle placement of 3 (range 1–5).
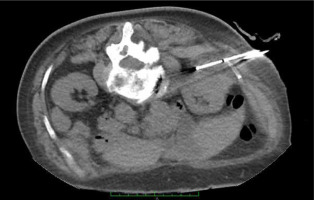
The vast majority of patients reported low intensity or no pain during the cryoablation procedure. During one case that was operated only under local anaesthesia, about one minute after the start of the cryoablation process the patient reported pain in an unclear loin location, and therefore the freezing procedure had to be ceased. The freezing was stopped and needles were activated one by one in order to localise the source of pain. On intra-operative CT, one of the needles (but not the iceball) was in contact with the lower edge of the 12th rib (Fig. 1).
The cryoprobe was realigned further away from the rib and the procedure continued. On her first follow-up visit, 6 weeks after the procedure, the patient reported intercostal neuralgia limited to the Th12 dermatome region, which started immediately after the procedure. This could be caused by irritation/damage to cutaneous branches of intercostal nerves.
The patients reported median perioperative nausea of 2 (range 0–8) on the 0–10 NRS. Three patients experienced significant nausea during needle placement and renal capsule irritation (scored as 8 on the NRS). It should be highlighted that the renal collecting system was not damaged in any of those patients. One of the patients required antiemetic administration.
In 5 patients, immediately after the cryoprobe removal, perirenal haematomas > 4 cm were visualised on follow- up CT. One of those was 10 × 5 × 3 cm. However, none of the patients presented clinically significant anaemia, nor required blood transfusions or additional procedures.
One patient experienced a temporary hypoesthesia of the right lower extremity, which corresponded with the side of the procedure. The symptoms subsided spontaneously after a few hours.
In one obese patient who was sedated with remifenta-nil, two (out of seven) cryoprobes slid out from the proper position before cryoablation onset (the procedure was for a cT1b lesion with the longest diameter of 5.4 cm). Deeper sedation was applied and the needles were repositioned. However, because of blood extravasation, the repositioning process was significantly more difficult. After the procedure, longitudinal (3 cm) thin wall blisters were found in the contact site. The patient was contacted one week later and reported that the lesion was healing properly.
Postoperative
Pain in the puncture site/lumbar area was a common ailment reported by the patients in the postoperative period. In the vast majority, the pain was of low/moderate intensity and in almost all cases did not require admini-stration of analgesics. Only 1 patient reported the use of tramadol for 2 days, while 2 others took paracetamol/non-steroidal anti-inflammatory drugs.
Fifteen (20.0%) patients reported general malaise of low intensity lasting a few days after discharge.
Six (8.0%) patients reported nausea and self-limiting vomiting on postoperative days 0 and 1 – probably asso-ciated with remifentanil sedation.
Five (6.7%) patients reported self-limiting macroscopic haematuria, which lasted for 2–5 days. One of these patients also reported the presence of small blood clots.
Four (5.3%) patients reported worse short-term blood pressure control. Three of them presented episodes of hypo-tension and one presented an episode of hypertension. Those deviations did not require long-term medication dosing adjustment.
Two (2.7%) patients reported a palpable “nodule” under the skin – one was operated on with a 14G Boston needle and one with a 10G IceCure probe.
Syncope occurred in 2 (2.7%) female patients on postoperative days 0 and 1. Both cases were connected with attempts to stand up.
Two (2.7%) female patients developed urinary tract infections 17 and 24 days after the discharge. Both of them underwent successful empiric antibiotic therapy in primary care without urine culture. As both of them were operated on with sterile urine and the infections started long after hospitalisation, a correlation was unlikely.
One (1.3%) patient was admitted 10 days after PCA because of fever and loin pain. The contrast CT revealed hydronephrosis and medium-sized urinoma around the operated kidney with no evident leak site. While performing cystoscopy with double-J stent (DJ) insertion, a 1 cm blood clot came out from the ureteral orifice followed by a squirt of cloudy urine. Urine extravasation was confirmed near the cryoablation site by a delicate pyelography, and a 6Fr DJ stent and a bladder catheter were inserted. Alongside wide-spectrum antibiotics, the patient was discharged on POD 2. DJ removal was performed after 6 weeks with no signs of further urine extravasation on contrast studies.
A small intraparenchymal renal artery pseudoaneurysm developed in 1 (1.3%) patient and was discovered at the 3-month follow-up CT. Being asymptomatic, it was not yet embolised.
In 50 (66.7%) cases the hospitalisation was uneventful and the patients did not report any toxicities during the first month after the procedure.
The complete SSQ-8 questionnaires were available from 30 patients (Table 3). The median SSQ-8 score was 9, indicating great satisfaction with the procedure. What is more, 93.3% of the patients answered that they would “definitely recommend” this surgery to someone else and 100% would undergo PCA again.
Table 3
Results of the Surgical Satisfaction Questionnaire (SSQ-8)
Discussion
In this article, we present a meticulous analysis of all complications and side effects of the PCA of renal tumours during the first 75 cases. The majority of the procedures were uneventful; to the best of our knowledge, only one patient required additional, minor surgical intervention.
According to the EAU guidelines, PCA is associated with shorter hospital stay than laparoscopic cryoablation [1]. Moreover, renal tumour ablation is a safe procedure, regardless of the ablation method (thermal/radiofrequency) or approach (laparoscopic/percutaneous) [1].
A recent meta-analysis found that PCA was associated with significantly lower overall and major complication rates when compared to PN [2]. The available literature reported that major complications occurred in 0% to 7.2% of PCA and 6.6% to 10% of PN procedures [4, 5]. This is in line with our data, as only 3 (4.0%) patients experienced more serious (Clavien-Dindo 3 or more) complications.
Pain
Although the process of cryoablation is painless, introduction of cryoprobes and the forced position can be associated with significant discomfort. Therefore, some patients might benefit from mild sedation and analgesia. Nonetheless, when compared to laparoscopic cryoablation and radiofrequency ablation, PCA procedures required significantly lower doses of analgesics [6, 7]. What is more, due to tissue damage or some amount of extravasated blood, pain may occur after PCA. In the present study, pain after PCA was the most common ailment. However, it is worth noting that the majority of patients reported a low/moderate intensity of pain, which usually did not require analgesics use. In a small study by Permpongkosol et al. [8], only 8% of the patients were administered analgesics after the PCA procedure.
Bleeding
In our study, in 5 patients large perirenal haematomas developed immediately after PCA, but they did not cause anaemia or require blood transfusions or any surgical interventions. Moreover, 5 (6.7%) patients reported haematuria (some with small blood clots), which lasted a few days. Finally, renal artery pseudoaneurysm developed in 1 patient, but did not require embolization. A recent analy-sis of 15 studies found that bleeding, including haematoma and haematuria, was the most common complication of PCA [4]. However, the authors also stated that minor bleeding is inevitable, due to renal anatomy and extensive vascularisation of RCC [4]. Nonetheless, the necessity of blood transfusion or arterial embolisation after PCA is rare and constitutes < 4% of all bleeding events [4]. According to the literature, risk factors of postoperative bleeding include advanced age, large tumour, higher number of cryoneedles and central tumour position [9]. It is worth mentioning that high-temperature ablation (radio-frequency or microwave) might have an advantage over PCA regarding bleeding risk. It is caused by the possibility of thermocoagulation during heat-based procedures [4]. A study on 573 renal ablations found that bleeding complications occurred in 7.2% of PCA procedures and just 1.2% of percutaneous radiofrequency ablations [9].
Malaise
Post-ablation syndrome is defined as mild fever and flu-like symptoms, such as malaise, myalgia, nausea and vomiting, which occur 24-48 hours after the ablation [10]. It is probably caused by cytokine release, due to necrosis of the tissues and tumour [11]. Zhong et al. [10] found that post-ablation syndrome was a frequent complication of the procedure, which afflicted 9% of the patients. According to the available data, malaise, nausea and vo-miting can occur in over 60%, 20% and 20% of the patients respectively [4, 12]. As for our experience, we observed a spectrum of symptoms that might correspond to post-ablation syndrome. Some patients, of whom the majority were sedated with remifentanil, reported general malaise, nausea and vomiting shortly after the procedure. However, none of them developed fever. Despite the above, the mean result of the SSQ-8 questionnaire indicates a high level of satisfaction with the procedure and the vast majority of patients answered that they would “definitely recommend” this surgery to someone else.
Blood pressure issues
Worse control of blood pressure after PCA might be caused by a number of non-kidney-related reasons, such as blood loss, dehydration, long-term prone position, analgesics, other drugs, stress, and many others. However, as the kidney plays a major role in blood pressure regulation, its injury during PCA might cause hypertension [13]. According to a study from 2017, intraoperative blood pressure was significantly elevated and was associated with the number of cryoprobes and endophytic extension, but not with pain [13]. Nonetheless, blood pressure after the procedure did not differ from the values before PCA [13].
Also, in some of our patients in the short follow-up we observed some benign problems with blood pressure control, with both hyper- and hypotension episodes.
Infections
In our series, we did not observe postoperative fever, but 2 cases of uncomplicated urinary tract infections developed a few weeks after PCA in patients with primarily nega-tive urine cultures. Although their correlation with PCA is doubtful, infections are a possible complication of any surgery, including PCA [4]. Moreover, the literature reported one case of PCA-related death, due to urosepsis [4]. In order to avoid life-threatening urinary tract infections, urine culture has to be performed prior to the procedure and antibiotic prophylaxis/treatment needs to be implemented according to the local protocols.
Skin lesions
A palpable “nodule” under the skin reported by 2 patients is not surprising, as PCA is performed using relatively large needles and minor scarring is possible. Also, hypoesthesia of the leg occurred in 1 patient directly after PCA. A lack of touch sensation and/or skin hyperesthesia is possible due to damage to the intercostal, lumbar, genitofemoral or lateral femoral cutaneous nerves [4].
Bowel
In the available, especially the early literature, descriptions of serious complications associated with bowel injuries may be found. According to the analysis by Iguchi et al. [4], 2.2% of PCA procedures were complicated with bowel injuries. However, only up to 0.6% required surgical resection. In our series, we were able to avoid those complications. It might be related to extremely meticulous patient positioning and needle placement regarding bowel location and extensive hydrodissection when in doubt. Also, in extreme cases, bowel safety was prioritised over the “radicalness” of the procedure.
Urinoma
PCA is associated with a lower risk of clinically signifi-cant urothelium damage when compared to classic ope-rations and other methods of focal treatment [14, 15]. The incidence is very low; however, urinoma might be a catastrophic complication that is extremely difficult to treat [16]. Therefore, meticulous cryoprobe placement and, in some cases, retrograde pyeloperfusion should be consi-dered [17]. In our population, we have observed one, clinically evident urinoma that required DJ placement and antibiotic therapy. The lesion healed well, with no further sequelae.
Others
A variety of other complications that were not observed in our population have been described in other research. Serious injuries to adrenal glands, pleura/lungs, spleen and liver are possible.
In our series, three patients required trans-liver puncture. However, the peri- and postoperative course was uneventful. Also, pulmonary embolism, cardio- and cerebrovascular events might develop as well as acute kidney injury and tumour seeding. Finally, even rarer events, such as catecholamine crisis and urinothorax, have been reported [4].
Deaths
PCA is associated with extremely low mortality. Even though we did not report any PCA-related deaths, in other studies the mortality rates reached up to 2.1% [4, 9, 18]. The causes of death were: pulmonary embolism, urosepsis, myocardial infarction, lung aspiration and sepsis [4, 18]. It has to be remembered that PCA is mainly performed as an imperative procedure in a severely comorbid population. Therefore, the risk of fatal events is significant, and not always directly associated with the ablation itself.
Limitations
There are some limitations of the present study that need to be disclosed. The included population was relatively small and the follow-up was short. Also, because of the low number of events, no statistical analysis could be performed. What is more, the included patients often presented major comorbidities. Also, anaesthesia and sedation methods were not homogeneous, which could have influenced the results. Finally, the procedure protocol was not uniform for every patient in terms of position as well as needle number and size. Also, some patients underwent periprocedural biopsy and/or hydrodissection.