Transcatheter aortic valve implantation or replacement (TAVI or TAVR) is a validated method of treatment of severe aortic stenosis, especially in patients at high surgical risk who are not suitable for surgical aortic valve replacement. Increased experience and improvements in implantation technique have led to a growing number of moderate- or even low-risk patients undergoing TAVI [1, 2]. However, the number of procedural complications remains significant, including new-onset or worsening cardiac conduction disturbances requiring permanent pacemaker (PPM) implantation. Except for broadly discussed factors contributing to PPM placement after the procedure, such as preprocedural right bundle branch block (RBBB), the use of self-expandable valve, or balloon post-dilatation [3], other potential predictors for the need of PPM implantation have been assessed including imaging risk factors. Moreover, predictors of PPM placement after TAVI in specific subgroups of patients were also investigated.
Calcification of the aortic valve and mitral valve annulus, left ventricular outflow tract calcification volume below the left and right coronary cusp, and porcelain aorta have been associated with PPM placement after TAVI, as mechanical compression of the cardiac conduction system by calcification seems to be responsible for the conduction disturbances after TAVI [3, 4]. Interestingly, calcification of the aortic knob or knuckle, being a part of the most distal part of the aortic arch [5], besides being easy to assess (Figure 1 A), may also have prognostic meaning. Significant aortic knob calcification (AKC) may be a marker of a widespread aortic calcification, and according to a study by Özderya et al. [6] it could be a predictor of post-TAVI PPM implantation (required in 15% of the investigated patients). The authors performed AKC grading using a 4-level system (from grade 0, indicating no calcification, to grade 3, defined as circular thickened calcification), based on posteroanterior chest radiographs. The multivariable regression analyses indicated that in 110 patients who underwent TAVI using a balloon-expandable TAVI system (Myval transcatheter heart valve, Meril Life Sciences, Gujarat, India), AKC was an independent predictor of PPM implantation after TAVI. The authors suggest that AKC assessment could be broadly used as a cost-effective, accessible, and easy to assess predictor of PPM placement after TAVI. This study highlights the importance of clinical assessment of aortic diseases and disorders [7, 8]. Importantly, the presence of porcelain aorta, which is defined by extensive calcification of the ascending aorta or aortic arch [8], may coexist with AKC in some patients. These risk factors, being dependent on the method of assessment, may indicate the same clinical entity. Moreover, AKC diagnosed based on chest radiograph might also overlap with enhanced aortic annulus calcification assessed with computed tomography (CT) or even mitral valve calcification seen on echocardiography (Figure 1 B). These hypotheses need to be tested in bigger, well-designed studies, possibly to establish superiority of one of these factors, especially with the use of various TAVI systems. This could lead to identification of the most valuable potential diagnostic and/or prognostic markers, as biomarkers in other cardiovascular diseases [9–13].
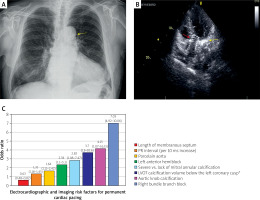
Figure 1
Transcatheter aortic valve implantation and the risk for permanent cardiac pacing. A – The posteroanterior chest radiograph before transcatheter aortic valve implantation indicating calcification of the aortic arch (aortic knob calcification, yellow arrow). B – Echocardiogram illustrating transcatheter aortic valve (red arrow) and calcification of mitral valve (yellow arrow) in the same patient, who required permanent cardiac pacing after valve implantation. C – Selected major electrocardiographic and imaging risk factors associated with permanent cardiac pacing after transcatheter aortic valve implantation. Aortic knob calcification data comes from multivariable analysis of a single, relatively small study, which indicated lower odds ratio in less complex multivariable model [6]; other data are based on [3] and respective studies cited by this paper. Above boxes indicating odds ratios, confidence intervals are provided. *> 13.7 mm3. It should be noted that left ventricular outflow tract (LVOT) calcification volume below the right coronary cusp > 4.8 mm3 was associated with odds ratio (95% confidence interval) of 4.7 (1.6–14.1), while LVOT calcification volume below the non-coronary cusp > 3.2 mm3 was not associated with permanent pacemaker implantation in a multivariable analysis [4].
An important anatomical factor related to PPM implantation after TAVI brought up by this study [6] is shorter membranous septum (MS), which is the upper part of the intraventricular septum. Shorter MS length is a risk factor for cardiac conduction disturbances after TAVI due to the proximity between aortic valve and the MS ‘separating’ it from the His bundle and bundle branches [14]. This association was also observed by Süygün et al. [15] among patients with bicuspid aortic valve (BAV) undergoing TAVI. Interestingly, in this study, which included 62 patients undergoing balloon-expandable transcatheter heart valve (Edwards SAPIEN XT, Edwards Lifesciences, Irvine, CA, USA) placement, all patients who required PPM placement (needed in 13%) had type 1 left-right coronary cusp fusion. Of note, there was a trend towards a need for PPM implantation in patients with the above-mentioned coronary cusp fusion morphology, although the tested difference did not reach statistical significance, probably due to the small study group. Süygün et al. showed that, similarly as in the general TAVI population (Figure 1 C), in patients with BAV, preprocedural RBBB is an independent predictor for PPM placement after TAVI. When pacing is needed, avoiding possibly harmful right ventricular pacing by conduction system pacing is promising also in patients after TAVI requiring PPM placement [3].
As shown above, there is an important need to find novel, strong, and independent predictors of PPM implantation after TAVI, as well as to validate preprocedural electrocardiographic and imaging factors suggested by recent studies. Research should also focus on novel methods of prevention of post-TAVI conduction abnormalities, considering the growing number of patients qualified for TAVI.








