Introduction
Currently, low-dose computed tomography (CT) approaches are routinely used for the physical examination of patients in a wide range of settings [1–3]. As low-dose CT screening for lung cancer has become more common, pulmonary nodule (PN) detection rates have risen significantly [1–3]. Reported rates of PN malignancy range from 5% to 70% [4, 5], and the diagnosis and treatment of lung cancer at an early stage can lead to significant reductions in patient mortality [6].
Video-assisted thoracic surgery (VATS) procedures are widely used to resect PNs [7]. To improve rates of VATS resection and to reduce rates of conversion to thoracotomy, preoperative localization is a common clinical strategy [8]. These localization procedures are performed using a variety of liquid and solid localization materials, the latter of which are not susceptible to diffusion away from the site of insertion and can be detected via palpation during VATS procedures [8]. Two of the most common solid localization materials are coil and hook-wire devices [8]. While coil localization is generally associated with lower rates of complications, the operative procedures necessary to successfully place these coils tend to be more complicated than those necessary for hook-wire localization [8, 9]. To reduce hook-wire localization complication rates while maintaining operative simplicity, anchored needle (AN) localization approaches based on the hook-wire strategy have been developed [10]. In contrast to hook-wire approaches, AN localization replaces the hook for an anchor-shaped device with four claws, providing superior stability. The anchor is also connected with smooth sutures in place of steel wire, which may provide patients with better comfort after localization is complete [10]. While there have been several studies to date comparing the clinical efficacy of hook-wire and coil localization strategies for patients with PNs [9], corresponding studies comparing the coil- and AN-based localization of PNs are lacking.
Aim
Here, the relative safety and clinical efficacy of CT-guided coil and AN localization approaches for patients with PNs were compared through a randomized controlled trial.
Material and methods
Our Institutional Review Board approved this single-center, prospective, open-label, randomized controlled trial, which was registered at ClinicalTrials.gov (NCT05183945), with all patients having provided written informed consent.
Patient selection
From January 2022 to July 2022, consecutive patients with PNs scheduled to undergo VATS procedures were randomly assigned to undergo preoperative localization using coil- or AN-based approaches. To be eligible for inclusion, patients needed to meet the following criteria: (a) patients with PNs, (b) PNs’ diameter > 5 mm, and (c) patients whose clinical and/or radiological findings were consistent with an intermediate-to-high risk of malignancy [4]. Patients were excluded from this study if any of the following were true: (a) they exhibited calcified PNs, (b) they exhibited PNs that grew smaller over the course of follow-up, (c) they exhibited typical metastases or any severe comorbidities; or (d) they declined trial participation.
CT screening was used to detect the PNs in all patients, with PN number, size, and location having been recorded.
Randomization
A block randomization strategy with a block size of 4 was used to randomize eligible patients into the coil and AN groups at a 1 : 1 ratio. Computer-generated random numbers were sealed within sequentially numbered opaque envelopes. A member of the Science and Education department who had no further role in this trial opened these envelopes before stent insertion.
CT-guided localization
Two interventional operators with more than 5 years of experience performing CT-guided interventional procedures performed all procedures with a 16-slice CT scanner (Siemens, Berlin, Germany) based on the following settings: 120 kV, 150 mA, and 1 mm thickness. Patient positioning was determined based on the locations of target PNs, while the selected needle pathway was the shortest path from the skin to the target PN, which allowed operators to avoid large blood vessels, ribs, and lung bullae. All localization was performed under local anesthesia.
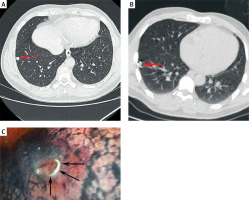
For coil localization, a fiber-coated coil that was 0.038 inches in diameter and 50 mm in length (Cook, IN, USA) was utilized. Using the appropriate needle pathway, an 18G coaxial needle (Precisa, Italy) was used to puncture the lung, advancing the tip of the needle until it was within 10 mm of the target PN. The coil was then inserted such that the tail of the coil remained visible [10], using the needle core to push the coil from the loading cannula into the needle sheath. After partially advancing the coil into the lung tissue, the needle sheath was smoothly removed such that the coil tail remained above the visceral pleura at a distance determined by the distance between the target PN and the pleura.
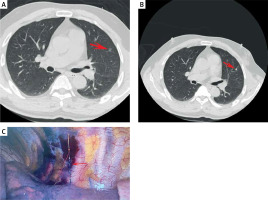
To perform AN localization, a 20G disposable PN localization needle 10 cm or 15 cm in length was utilized (Senscure, Ningbo, China). The procedures for needle puncture were the same as those for the coil group. After the tip of the needle had reached the target position, the safety buckle was removed and the pusher was depressed such that the anchor was released in the lung parenchyma around the target PN. Following the subsequent removal of the needle, a tri-colored suture marker remained visible within the needle pathway, with the distal end of the suture remaining outside of the pleura.
In patients undergoing localization of multiple PNs, all target nodules were detected with a one-stage CT-guided localization procedure. Post-localization CT scans were used to confirm the locations of the chosen localization materials and to evaluate any potential localization-related complications in all patients.
VATS procedures
Routine VATS-guided wedge or segmental resection procedures were performed under general anesthesia within 3 h after localization was complete. During these procedures, the utilized localization materials could be readily identified by surgeons and palpated by hand as needed. Surgeons were able to confirm target nodule positions based on the distance between these nodules and the target localization marker upon CT examination. Based on the distance between the pleura and the target nodule, wedge or segmental resection was performed using a cutting suture device. All resected samples underwent the analysis of intraoperatively collected frozen pathology sections. When invasive lung cancer was diagnosed by this approach, patients underwent additional lobectomy and lymphadenectomy. In all other instances, lobectomy was not conducted. Lymph node sampling was conducted for patients diagnosed with mini-invasive lung cancer.
Definitions and endpoints
CT-guided localization technical success was defined by the successful visualization of the coil/AN during VATS resection without the dislodgement of these materials, together with the successful removal of the target PN within the resected portion of the lung parenchyma. CT-guided localization procedural duration was measured as the interval from the first to the last CT scan. A visual analog scale (VAS; 0–10 points) was used to assess patient pain severity. The VATS procedural duration was measured as the interval from the first incision to wound closure.
CT-guided localization technical success rates were the primary study outcome, while secondary outcomes included the duration of localization, VAS scores, the incidence of localization-related complications, VATS procedural duration, VATS type, and final patient diagnoses.
Statistical analysiss
This was developed as a noninferiority trial, with initial estimates of a technical success rate of 97% for localization procedures performed in both groups of patients [8, 10]. Differences in the rates of technical success between these two localization strategies were assessed with a -10% noninferiority margin based on a one-sided significance level of 0.025. Using these calculations, a minimum of 100 patients (n = 50/group) was deemed necessary to have 80% statistical power necessary to demonstrate noninferiority when accounting for an approximate 10% dropout rate.
The present study was conducted using an intention-to-treat analytical approach based on all enrolled patients. Continuous results that were normally distributed were reported as means ± standard deviations, whereas they were otherwise reported as medians (Q1; Q3), and they were compared with Student’s t-test or the Mann-Whitney U test. Categorical data were compared with the χ2 test or Fisher’s exact test. A logistic regression approach was used to compare risk factors associated with localization-related complications. P < 0.05 served as the threshold for significance, and all statistical analyses were performed in SPSS 16.0 (SPSS, Inc., IL, USA).
Results
Patients
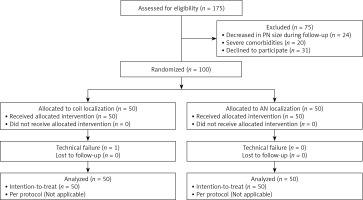
This study ultimately enrolled 100 patients with 120 PNs who were randomly assigned to the coil (patients = 50; PNs = 60) and AN (patients = 50; PNs = 60) groups. Patient selection-related details are presented in Figure 1, while baseline patient data are presented in Table I. With the exception of the distribution of the nature of these PNs, all other data were comparable between these groups.
Table I
Comparison of baseline characteristics between groups
PN localization
The respective technical success rates for localization procedures performed in the coil (Photo 1) and AN (Photo 2) groups were 98.3% (59/60) and 100% (60/60), with no significant difference between these groups (p = 1.000, Table II). The one instance of technical failure in the coil group was the result of the entire placement of the coil. Coil/AN dislodgement did not occur in any case. The median localization procedure duration in the coil group was significantly longer than that in the AN group (16.0 vs. 8.0 min, p < 0.001). There was no significant difference in VAS scores between these groups (3.4 ±0.6 vs. 3.4 ±0.6, p = 0.715).
Table II
Comparison of localization-related data
Localization-related complication rates
Pneumothorax impacted a total of 5 (8.3%) and 3 (5.0%) patients in the coil and AN groups, respectively, with the incidence rate being comparable in both groups (p = 0.715). None of these instances of pneumothorax required further medical intervention, nor did they interfere with the VATS procedure. Based on logistic regression analyses, a longer localization procedural duration was independently associated with a greater risk of pneumothorax (p = 0.034, Table III).
Table III
Predictors of pneumothorax after localization
Pulmonary hemorrhage impacted a total of 3 (5.0%) and 8 (13.3%) patients in the coil and AN groups, respectively, with no significant difference between these groups (p = 0.110). Pulmonary hemorrhage did not interfere with VATS procedures in any case, and logistic regression analyses failed to identify any factors significantly associated with the risk of pulmonary hemorrhage.
VATS resection
Technical success rates for limited VATS resection procedures were 100% in both the coil and AN groups (Table IV). Even in the one instance of technical failure in the coil group, VATS wedge resection was performed successfully as the operator was able to palpate the coil intraoperatively. A similar median VATS procedural duration was observed in the coil and AN groups (100 min vs. 85 min, p = 0.549). For details regarding surgery types and pathological diagnoses (Table IV). Of the patients diagnosed with invasive adenocarcinoma, three (1 in the coil group, 2 in the AN group) did not undergo subsequent lobectomy as they lacked sufficient pulmonary functional reserve.
Table IV
Comparison of VATS related data
Discussion
Preoperative CT-guided localization is widely used in patients with PNs scheduled to undergo VATS resection, as it can facilitate a significant reduction in the need for conversion to thoracotomy [11]. These preoperative localization strategies can also reportedly decrease VATS procedural duration and stapler utilization [11]. An effective localization marker should exhibit good stability, ease of implementation, soft consistency, and ease of visualization during VATS procedures. Coil localization offers advantages including the stability of the coil in the lung parenchyma owing to its ring-shaped construction, the ease of coil tail visualization during VATS procedures owing to it remaining on the pleura, and the low rates of associated complications as the coil does not penetrate the thoracic cavity. However, coil localization necessitates a high degree of technical skill, and the ring-like shape of the coil can lead to it accidentally being fully inserted within the lung parenchyma in some cases [11].
Comparable rates of technical success were observed for both tested localization strategies (98.3% vs. 100%, p = 1.000), with no patients having experienced coil or AN dislodgement. This suggests that AN materials exhibit similarity similar to those of coils. However, the AN approach did reduce the procedural duration relative to coil localization (8.0 vs. 16.0 min, p = 0.001), potentially because this localization strategy is simpler [10, 12].
Here, the safety outcomes in the coil and AN groups were comparable, with similar rates of both pneumothorax (8.3% and 5.0%) and hemorrhage (5.0% and 13.3%) for patients who underwent either of these localization procedures, and with these rates being lower than those reportedly associated with hook-wire localization (35% and 16%) [13]. In logistic regression analyses, longer localization duration was independently associated with pneumothorax risk, consistent with what has been reported previously with respect to CT-guided localization or biopsy [14–16]. No risk factors associated with pulmonary hemorrhage were identified in this study, potentially owing to the small sample size.
As both the coil and AN localization materials can be readily visualized while performing VATS procedures, both can be used as an effective means of guiding VATS limited resection, although the resection depth should be confirmed in the coil group with reference to preoperative CT images [17]. AN localization, in contrast, utilizes a tri-colored marker suture that can provide direct confirmation of resection depth for the target PN [18, 19]. While there was no significant difference in VATS duration between these groups (p = 0.549), median VATS duration was shorter in the coil group than in the coil group (85 vs. 100 min).
In the present study, lobectomy was required in 28.3% of PNs owing to the presence of invasive adenocarcinoma, whereas in the remaining cases, limited wedge or segmental resection was sufficient as a surgical approach. As such, adequate preoperative localization is an effective approach to preserving lung function in treated patients.
There are certain limitations to the present study. For one, while a block randomization approach was employed, this randomization was not stratified according to the nature of patient PNs. Imbalance in the nature of PNs between groups may have contributed to some degree of selection bias. The overall sample size was also fairly limited. Lastly, this was a single-center study, so the results warrant further multi-center validation.