Introduction
Fungal infection is the most predominant superficial infectious disease that affects the skin surface, nails, hair, and subcutaneous tissues under the skin. Appearing on the surface of the skin as a commensal, Candida albicans is the major infectious agent that affects human skin (Garber, 2001; Guégan et al., 2016). Candidal infection results in skin rashes, red itching, and inflammation that can spread all over the body and is rapidly transmitted from infected persons to healthy ones. It mainly affects wet skin and damp and furrowed areas, such as subarms and intergluteal areas (Kuhbacher et al., 2017). Upon infection, C. albicans damages the skin surface initially, and later, it invades deep dermal layers through desquamation, causing subcutaneous mycosis. If the host immune system is weak, it can also invade deep tissues and cause a wide range of life-threatening neutropenia conditions (Ameen, 2010; Havlickova et al., 2008; Queiroz-Telles et al., 2003; Zhang et al., 2007).
Reports published in 2020 indicate that about 40 million individuals in underdeveloped and developing countries were affected by fungal infection (Coates et al., 2020). Researchers around the world are actively investigating the application of antifungal chemotherapies to treat surface and deep fungal infections (Ansari et al., 2019; Dave et al., 2017; Garg et al., 2020; Gupta and Cooper, 2008; Wingard and Leather, 2004). Resistance to antifungal drugs has increased dramatically in C. albicans, resulting in reduced effectiveness of treatment for Candida infections (Heredero-Bermejo et al., 2020; Zamani et al., 2020). Topical therapy is a conventional systematic treatment for fungal infections (Alqahtani et al., 2020; Zhang et al., 2007). When topical treatment is performed directly on the infected site, a significant reduction in disease symptoms is observed (Deyno et al., 2019; Kowshihan et al., 2020). However, the stratum corneum, the outer layer of the skin, is the critical barrier to drug permeation. Therefore, for an antifungal drug to be effective, it should have a drug delivery system that can pass the barrier of high mechanical strength to the subcutaneous tissue (Khalid et al., 2021; Ré et al., 2021).
However, topical application of antifungal drugs can trigger adverse reactions in the skin such as allergic reactions and itching (Dismukes, 2000; Naik et al., 2000; Silva et al., 2014). Although more synthetic antifungal formulations are commercially available nowadays, such as creams, gels, and lotions, they are not suitable for pharmaceutical applications due to poor absorption and other adverse effects. New drug delivery mechanisms are being developed to overcome these challenges and to develop a topical cold cream formulation with reduced local side effects and increased therapeutic effectiveness (Rapalli et al., 2020; Sonia et al., 2017; Ugazio et al., 2020). Cold cream is an excellent face cleanser, and it protects the skin against environmental stress; sometimes, it is used as a topical drug carrier for pharmaceutical applications (Sonawane et al., 2021; Sonia et al., 2017).
Caralluma adscendens var. attenuata grows in dry deciduous forests and scrub jungles (Kumar et al., 2014) and is a good source of steroids and glycosides (Kiranmayee et al., 2016). Caralluma exhibits effective antioxidant, anti-inflammatory, antidiabetic, and antimicrobial activities and so can be used in pharmaceutical applications (El-Shiekh et al., 2021; Madasamy et al., 2020; Vajha et al., 2010). Therefore, a cold cream based on the extract of C. adscendens var. attenuata was formulated in this study to treat C. albicans infection and tested on the rat skin under laboratory conditions.
Methods
Extraction and preparation of Caralluma cold creams
Cladodes of C. adscendens var. attenuata were collected in June around Sivakasi (latitude: 9.463898°N 77.760829°E), Tamil Nadu, India. The collected plants were verified, and their identification was confirmed by comparing with an authentic specimen by a taxonomist at the Centre for Research and Postgraduate Studies in Botany, Ayya Nadar Janaki Ammal College, Sivakasi, Tamil Nadu, India. The collected plant cladodes were dried in a shadow for a week and then finely powdered. Then, 100 g of the powdered plant material was subjected to 12 h extraction in 600 ml of 95% methanol (AR grade) using the Soxhlet apparatus. The supernatants thus obtained were evaporated and dried in a vacuum under reduced pressure at 55–60°C. Then, the residues were collected and stored at 4°C for further use.
Cold creams (water/oil emulsion) were prepared according to the procedure of Carter (2008) with slight modifications. The basic composition of the cold creams is presented in Table 1. The oil-phase components such as beeswax, paraffin wax, cetyl alcohol, and mineral oil were taken in a beaker, mixed, and heated in a water bath at 70°C for 10 min. Simultaneously, borax, methanolic extract of C. adscendens var. attenuata, and sterile distilled water were mixed in another beaker and heated up to 70°C for 10 min, which formed the aqueous phase. After heating for 10 min, the aqueous phase was added slowly to the oil phase with continuous stirring until a uniform smooth paste was formed. The prepared cream was packed in a sterile plastic container and stored in a refrigerator at 4–8°C for further use.
Table 1
Basic composition of cold cream formulation using methanolic extract of Caralluma adscendens var. attenuata
Evaluation of physical appearance and visual examination of the cold creams
The following characteristics were evaluated in the formulated creams: color, texture, homogeneity, stability, pH, spreadability, viscosity, and in vitro permeation.
Determination of pH
pH was measured in triplicate for each cold cream using a digital pH meter (Hanna-98107, India).
Homogeneity
The homogeneity parameter determined the consistency of the cream. It was tested manually by pressing a small quantity of the formulated cream between the thumb and index finger. Immediate skin feel (such as stiffness, grittiness, and greasiness) was evaluated.
Spreadability test
Spreadability of the formulations was evaluated by measuring the spreading diameter of 300 mg of the sample after placing it for 1 min between two horizontal glass plates (glass plate size 7.5 × 2.5 cm). The weight of the top plate was 20 g. Each formulation was tested in triplicate (Haneefa et al., 2010). The results were recorded, and spreadability was calculated by using the following formula:
where S is spreadability, m is the weight tied to the top slide, l is length (−7.5 cm), and t is time (s).
Stability studies
Stability tests were performed in compliance with the International Council on Harmonisation criteria to determine drug and formulation stability (Bajaj et al., 2012). The cream sample was filled in a glass bottle and kept under accelerated conditions of 40 ± 2°C/75± 5% relative humidity (RH) in a programmable environmental test chamber (Remi CHM-16S) for 90 days. Samples were taken at the end of the experiment, and physical properties and viscosity were evaluated.
Viscosity measurement
Viscosity of the formulated creams under different shear rates was calculated using the Brookfield viscometer (Spindle type, S-24; LVDV-E model) in the range of 10–100 rpm (revolutions per minute) at 25°C. A sample of 20 g of cream was taken in a beaker, the spindle was dipped into the beaker for around 5 min, and then, the reading was taken.
In vitro skin permeation studies
In vitro skin permeation tests were performed using a dialysis membrane (AB-253a, Abron Exports, India) of pore size 10–100 kDa. To create a uniform surface to place the transdermal patch, the membrane was connected with one side and its other side was an openended cylinder. The side where the transdermal patch resided was considered the donor compartment. The membrane’s whole surface is in contact with the receptor compartment containing 150 ml of phosphate buffer (pH 6.8) and maintained at a temperature of 37 ± ± 2°C. A magnetic stirrer was used to stir the contents of the receiver compartment at a constant speed of 100 rpm. Two milliliters of the sample was collected from the receiver compartment every hour up to 8 h, and an equal quantity of fresh receptor medium was added every time to maintain the volume of the content in the receiver compartment constant. Then, the antioxidant activity of the contents in the receptor compartments was tested using DPPH. This experiment was performed in triplicate, and the results were presented as the mean ± standard deviation (Mahdi et al., 2011).
Animals
Thirty albino Wister female rats (170 ± 10 g) were obtained from an animal house, and the experimental procedure used in this study was approved by the Institutional Ethics Committee of K. M. College of Pharmacy, Madurai, Tamil Nadu, India. The Committee approved the study protocol (clearance no.: 661/PO/Re/S/02/ CPCSEA) based on the control and supervision of experimental animal guidelines in India. The animals were then divided into six groups (n = 5/group), housed in polypropylene cages under aseptic conditions, and fed with standard pellet and water ad libitum.
Skin irritation test
The skin irritation test was carried out on albino rats as follows: the animal fur was shaved from the backside in an area of 3 cm2. The first rat group served as control, in which 5% sodium lauryl sulphate in distilled water was applied; in the second group, standard plain cream was applied; and in the remaining three groups, FCA 50 mg, FCA 100 mg, and FCA 200 mg (concentrations of the prepared cold cream) were applied (Hiremath et al., 2008). A pinch of cream was rubbed on the rats’ skin twice a day for 3 days. The experiments were performed in triplicate to evaluate the morphological changes on the skin surface.
Preparation of immunosuppressed animals
Immunosuppressed albino Wister rats were used for testing the effect of the prepared formulations in preventing and treating deep fungal skin infections in the case of extreme skin infections. The rats were housed in individual cages and allowed to receive food and water ad libitum. To suppress rat immunity, cyclophosphamide (150 mg/kg × 3 days) was intraperitoneally injected (Aldawsari et al., 2015; Gupta and Vyas, 2012).
Fungal strain preparation
Candida albicans MTCC7315 (obtained from MTCC – Microbial Type Culture Collection and Gene Bank, Chandigarh, India) was inoculated in potato dextrose broth (Hi-Media, Bangalore, India) and allowed to grow at 30°C for 48 h. The fungal cell pellets were collected via centrifugation after incubation, washed twice with PBS and 70% ethanol, and resuspended in PBS solution.
Infusion of candidal infections and cold cream treatment
To study the effects of the prepared topical creams on fungal infections, the animals were divided into six groups (n = 5/group): group 1, left untreated and considered control; groups 2–6 were subjected to skin candidiasis by means of C. albicans. Each rat from groups 2–6 was injected intraperitoneally with 100 μl of 107 CFU/ml C. albicans cell suspension in the middle of the shaved region, following the procedure of Qushawy et al. (2018). The injected site was rubbed until the tiny edema had vanished and were observed for 72 h. After 72 h, 300 mg of the appropriate cream formulation was applied on the infected rat skin twice a day and rubbed, following the protocol of Qushawy et al. (2018). Group 2 served as a control for C. albicans infection without treatment, group 3 was treated with plain cream, group 4 was treated with FCA−50 cream, group 5 was treated with FCA−100 cream, and group 6 was treated with FCA−200 cream. After 7 days of treatment, the skin condition was observed and photographed.
Microbial count
After completion of the treatment, the rats were anesthetized, and the infected skin samples were collected, washed, spread-plated onto potato dextrose agar medium (Hi-Media, Bangalore, India), and incubated for 48 h at 37± 1°C. The viable values of colony-forming units (CFUs) were then recorded, and all the results were presented as mean ± standard deviation (Aldawsari et al., 2015).
Histopathology analysis
At the end of the experiments, the rats were anesthetized, and the infected skin was collected, sliced, fixed with 10% formaldehyde, and then paraffin-blocked. Then, slides were made from paraffin blocks and dyed with hematoxylin–eosin, all according to the procedure of Abdellatif et al. (2020). The slides were visualized using an optical microscope (40 ×; Olympus microscope, CH20iBIMP with micro view × 86 Software), which showed signs of inflammation and epidermal and dermal changes.
Statistical analysis
One-way analysis of variance with post hoc comparison was used to investigate the significant difference between results. P < 0.05 was considered statistically significant. All statistical analyses were carried out using SPSS package, version 21.0 (IBM corporation, Armonk, New York).
Results and discussion
The efficacy of the C. adscendens var. attenuata extract cold cream was evaluated ex vivo in albino Wister female rats, after inducing skin infection with C. albicans. All the prepared formulations were evaluated for physical appearance, homogeneity, stability, pH, spreadability, viscosity, in vitro permeability, and skin irritation.
Physical appearance and stability
All the formulated creams were semisolid, with a pale green to dark green color depending on the concentration of the extract, and the plain cream was white (Table 2). All these creams showed consistent texture with good homogeneity and without lumps. Therefore, the formulated creams showed good topical applicability (Chandrasekar et al., 2018).
Table 2
Evaluation of physical parameter studies of prepared C. adscendens var. attenuata cold cream
Stability studies
A herbal formulation’s quality, safety, and efficacy for commercialization are dependent upon its stability. Thus, stability studies are essential for the development and improvement of formulations by establishing their validity and monitoring their physical and chemical properties (Burki et al., 2020). For stability analyses, all the prepared formulations (FCA−50, FCA−100, FCA−200, and plain cream) were kept at a temperature of 40± 2°C and RH of 75 ± 5% for 90 days, and the time required for phase separation and changes in color, pH, homogeneity, and spreadability were recorded. Before stability studies, all the formulated creams showed a smooth texture, and phase separation (oil/water) was not observed (Table 2). After 90 days of stability studies, all the formulated creams were still stable under 40°C + 75% RH, and no phase separation or color change was observed. These results demonstrated that the formulations were relatively stable under accelerated temperature and humidity conditions.
pH value
The pH of all the prepared formulations was tested. The plain cream showed a pH value of 6.1; for FCA−50, FCA−100, and FCA−200, the pH values were 6.1, 6.22, and 6.3, respectively, which were acceptable for skin formulations (pH ranging from 4 to 6, according to Lukić et al., 2021). The pH of all the formulations was analyzed at 0, 30th, 60th, and 90th days. Interestingly, slight variations in pH were observed during 90 days of storage, but they were within the normal skin pH range (Table 2). Throughout the experiment, the pH of the formulated products was within the acceptable range for dermal preparation.
Spreadability
Spreadability analyses of the formulated creams indicated that all the prepared formulations were evenly and easily spread on the skin surface (Khan et al., 2020). As summarized in Table 2, FCA−200 exhibited the highest spreadability calculated by means of the time required by the top slide to slip over the bottom slide (12.62 ± 0.5 s; the lower, the better). In comparison, the plain cream showed a spreadability of 18.6 ± 0.5 s. Interestingly, FCA−50 and FCA−100 showed a spreadability of 13.32 ± 0.5 and 12.74 ± 0.5 s, respectively. Consistent with this study, Ijaz et al. (2021) reported that the spreadability of the formulations containing herbal cosmetic cream of Aloe vera gel and tomato powder was in the range of 9–13 s, which was in the acceptable range. As mentioned earlier, spreadability plays an essential role in administering a medicated formulation to the skin and also in the efficacy of the formulation (Dhyani et al., 2019).
Viscosity
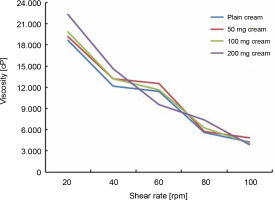
Viscosity refers to the stickiness or thickness of a semisolid cream and is the most important quality control parameter of the formulated creams. All the tested creams showed gradual changes in viscosity with an increase in rpm. FCA−50 and FCA−100 showed a change in viscosity from 19 153 to 4895 and from 19 850 to 4139 cP, respectively. Based on these values, both FCA−50 and FCA−100 creams had a similar viscosity curve. FCA−200 showed a higher viscosity, ranging from 22 345 to 3857 cP, and the plain cream showed the lowest viscosity, ranging from 18 738 to 4365 cP (Fig. 1). Viscosity is considered crucial as it defines the shelf-life stability, product esthetics such as clarity and ease of flow of the product package, and the spreadability of the cream on the skin (Alquadeib et al., 2018; Khan et al., 2020).
In vitro permeation test
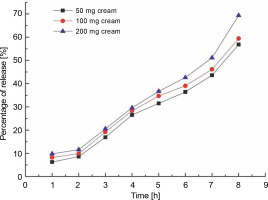
The most essential step in designing a successful topical therapy is maintaining sufficient permeability of drugs through the skin (Pithayanukul et al., 2002; Sequeira, 1990). Permeation analysis was used to evaluate the time taken for the sustained release of the drug across the dialysis membrane. The data obtained for permeation analysis of the prepared samples are shown in Figure 2. The time necessary for the release of the formulated creams across the membrane was calculated as a percentage of the same value obtained for the control sample. When compared with the control group, FCA−50 showed a gradual increase from 6.2 to 56.8%, FCA−100 from 8.2 to 59.3%, and FCA−200 from 9.7 to 69.3% after the first and eighth hours of suspension in the apparatus. According to a prior study, a sertaconazole nitrate-loaded nanovesicular gel with high drug penetration offers significant antifungal activity (Abdellatif et al., 2017). Similar to the creams formulated in the present study, the sertaconazole nitrate-loaded nanovesicular gel exhibited a high degree of permeability. Thus, the in vitro skin permeation test accurately predicts the result of an in vivo experiment and offers a credible estimate of bioequivalence.
Skin irritation study and treatment with cold cream formulations
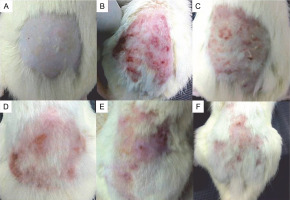
The formulated cold creams were non-irritant and did not cause adverse effects on the applied area after 72 h of the study, indicating that they were safe for the skin. The antifungal properties of the prepared cold creams were tested by inhibiting C. albicans infection of the epidermal skin of the studied rats. Candida albicans mycoses induced on the skin surface appeared reddish with lesions on the extensor surfaces and coarse blisters (Fig. 3). The formulated cold creams, when applied on the infected skin region, reduced the C. albicans infection. A high reduction in skin fungal infection was observed after 7 consecutive days of topical application of 200 mg FCA (i.e., in rats from group 6). Topical application of 50 and 100 mg FCA showed moderate effects in inhibiting the fungal infection (i.e., in rats from groups 4 and 5, respectively). No inhibition of skin infection was reported in groups 2 and 3 (the control group for C. albicans infection and the group that received the plain cream, respectively, Fig. 3). Candida albicans initially invades the epidermal skin layer and suppresses or alters the immune response to stimulate lower cytokine response, thus causing severe damage (Mukaremera et al., 2017; Netea et al., 2015). We demonstrated that C. albicans caused morphological changes in rat skin similar to those in human skin (Hube et al., 2015; Schaller et al., 2000). We showed in our previous report that terpenes and essential oils were present in the methanolic extract of C. adscendens var. attenuata triterpenes (Sundar et al., 2021). A previous study has also shown that these plant metabolites are active antimicrobial compounds (Espino et al., 2019). The mechanism of action of this class of compounds is speculated to involve fungal membrane disruption and fungal mitochondrial destruction (Lagrouh et al., 2017; Tian et al., 2012). In a previous study in which a promising natural compound in different topical cream formulations of C. albicans was tested, a reduction in the total fungal load was observed 6 days after the treatment was started (Zinsou et al., 2020). Moreover, Campos et al. (2018) found that the herbal formulation of the Mitracarpus frigidus extract showed acceptable physicochemical and potent antifungal properties against C. albicans skin infections.
Fig. 3
Images showing experimental animals initially infused with 100 μl of 107 CFU/ml of Candida albicans ; after 72 h, 300 mg of Caralluma adscendens var. attenuata cold cream was applied on specific skin surfaces; A) group 1, B) group 2, C) group 3, D) group 4, E) group 5, and F) group 6; at the end of the treatment, on the dorsal skin of each rat, the occurrence of patches, spots, and hair growth was noted, and the skins were photographed
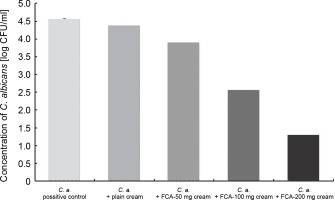
The microbial count is essential in the determination of infection ranges in the skin samples (Gravning et al., 2021). CFU was calculated in the skin of all the rat groups; groups 2 and 3 showed well-defined infections, with the microbial count of 4.55 and 4.36 log10 CFU/ml, respectively. The post hoc comparison revealed that group 6 (i.e., treated with 200 mg FCA) showed a significant decrease in the CFU value of the infected site to 1.28 log10 CFU/ml, compared with control samples (P < 0.05) (Fig. 4). Groups 4 and 5 (i.e., FCA−50 and FCA!100 groups), showed CFU values of 3.88 log10 and 2.53 log10 CFU/ml, respectively, indicating a slight decrease in the infection severity, compared with plain cream treatment (group 3). Based on these results, FCA−200 cream showed a high therapeutic efficacy as it resulted in significant fungal colony eradication in the skin epidermal layer, which is consistent with the previous report (Bonifácio et al., 2019), where the antifungal activity of Astronium urundeuva was observed in suppression, germination, and inhibition of mycelial growth in female Wistar rats. The high antifungal activity of FCA−200 could be due to the presence of numerous active compounds in the cold cream, such as triterpenes, terpenes, and essential oils, as reported in a previous study (Sundar et al., 2021).
Histological observation of skin
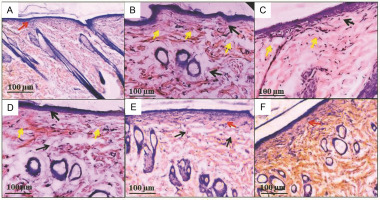
Histopathological analysis is a quick and inexpensive way to diagnose invasive fungal infections. The uninfected skin tissue is arranged into uniform dermis and epidermis layers, with the basal cells lining the base and spinous keratinocytes with notable intracellular structures overlaid (red arrow, Fig. 5A). Extensive skin damage and abnormalities were observed after the induction of C. albicans infection. At the same time, fungal hyphae were noted in the superficial epidermal cells (yellow arrow, Fig. 5B), as well as the focal interface dermatitis (black arrow) and dense and chronic hyperkeratosis of the dermis layer (black arrow, Fig. 5C), which is in agreement with a previous report indicating that Candia spp. affected the epidermal and epithelial tissues (Gácser et al., 2007). By contrast, in group 6 (rats treated with 200 mg FCA cream), effective regeneration of the epidermal cell structure with a mildly compact hyperkeratosis layer was observed, compared with groups 4 and 5 (rats treated with 50 and 100 mg FCA). Groups 4 and 5 showed a marked reduction in cell inflammatory infiltration, which remained mostly in deep layers of the lamina propria (Fig. 5D–F). A previous study demonstrated that plant-based (M. frigidus) formulations have mitigated the pathological leukocyte infiltration and high tissue reproducible capacity in candidal infections (Campos et al., 2018).
Fig. 5
Histopathological observations of the epidermal skin layer; Candida albicans induced epidermal damage, and the treatment with formulated Caralluma adscendens var. attenuata cold cream inhibited the epidermal damage; hematoxylin and eosin staining of rat dorsal skin under (40 ×) view; scale bar: 100 μm; A) group 1, B) group 2, C) group 3, D) group 4, E) group 5, and F) group 6
Conclusions
The results of this study indicate that the cold cream formulations developed based on the methanolic extract of C. adscendens var. attenuata showed antifungal activities against C. albicans in rat skin. These findings suggest that the prepared cold cream could be effectively used to treat fungal infections without damaging the skin surface and also that the topical formulation C. adscendens var. attenuata cold cream showed good stability and permeability, with no signs of irritation. The prepared cold cream formulations were tested against C. albicans infection and found to be helpful in reconstituting the original structure of the dermal layer in the subcutaneous layer of the skin.