Summary
Aortic stenosis (AS) results in left ventricular outflow tract obstruction, potentially leading to heart failure development. Moreover, iron deficiency (ID), common among elderly heart failure patients, can cause symptoms similar to AS. Despite the clinical benefits of transcatheter aortic valve implantation (TAVI), some patients do not experience improvements in exercise capacity. This raises the question of whether iron deficiency could be related to the lack of improvement after the procedure. This study found no significant difference in the 6-minute walk test between ID and non-ID patients following TAVI. ID likely has no impact on physical capacity in patients with severe AS.
Introduction
Aortic stenosis (AS) causes left ventricular outflow tract obstruction, which may progress to heart failure (HF) and ultimately results in a poor prognosis. Its prevalence in the over-65 population ranges from 2% to 7% and increases with age. Despite its undisputed clinical benefits and usefulness among AS patients, transcatheter aortic valve implantation (TAVI) is associated with high intermediate and long-term mortality rates, as well as a lack of functional improvement in a certain number of patients [1–4]. Previous studies have confirmed that not all patients experience improved exercise capacity following a successful TAVI procedure. It is estimated that lack of improvement in the 6-minute walk test (6MWT) distance occurs in up to 30% of patients after valve implantation [5].
At the same time, iron deficiency (ID) is common in the population of elderly patients particularly burdened with HF. In patients with HF, intravenous iron administration has been shown to enhance exercise capacity, improve quality of life, optimize myocardial function, and decrease the likelihood of hospitalization for heart failure [6, 7]. It is noteworthy that the symptoms of ID, such as fatigue, shortness of breath, and reduced exercise capacity, are similar to the main symptoms of symptomatic AS [8, 9]. This raises the question of to what extent ID may, if diagnosed, be related to the lack of improvement in physical performance after TAVI. This question is all the more pertinent because ID supplementation is relatively simple and inexpensive, providing an opportunity for potentially cost-effective treatment to improve outcomes after TAVI.
Aim
This study aimed to assess the prevalence of ID in a group of patients scheduled for TAVI and to evaluate the impact of this parameter on 6MWT results before and after the procedure.
Material and methods
Consecutive patients with severe AS, selected for TAVI by the decision of a multidisciplinary Heart Team in the Institute of Heart Diseases, Wroclaw, Poland, were screened for iron deficiency.
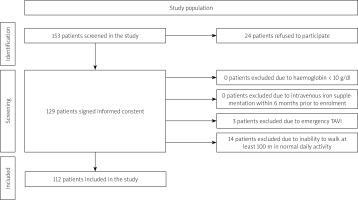
Exclusion criteria included haemoglobin < 10 g/dl, intravenous iron supplementation within 6 months prior to enrolment, emergency TAVI, and inability to walk at least 100 m in normal daily activity. All study participants included in the study provided informed consent approved by the local ethics committee. The research adheres to the ethical guidelines of the 1975 Declaration of Helsinki as evidenced by prior approval by the institution’s bioethics committee.
Before the TAVI procedure, all patients underwent assessment of NYHA class, 6MWT, and laboratory tests including markers of iron homeostasis (ferritin (μg/l), serum iron (μg/dl), total iron binding capacity (TIBC, μg/dl), and transferrin (mg/dl)). Serum ferritin levels were determined using an immunoassay based on electrochemiluminescence methods, while serum iron and TIBC were assessed using a substrate method. Transferrin saturation (TSAT) was reported as a ratio of 0.7217 × serum iron and transferrin, multiplied by 100. Iron deficiency was defined as a ferritin level < 100 μg/l or in the range 100–299 μg/l along with TSAT less than 20%. Patients meeting those criteria were classified as the iron deficiency (ID) group, while patients with normal laboratory values formed the non-ID group.
In addition, all patients were tested for peripheral blood reticulocyte haemoglobin content (Ret He) using automated blood cell analysers, with 30 pg considered the lower normative limit.
The 6MWT was conducted following the American Thoracic Society guidelines, which outline the methodology, indications, contraindications, and practical recommendations to ensure test quality and reproducibility [10]. The test took place in a four-metre-wide corridor with a firm, even floor, and the total walking distance was measured after 6 min. Before the test, patients rested in a seated position for 10 min. Patients were instructed to swiftly walk for 6 min or until dyspnoea or muscular fatigue occurred. The walking course covered 30 m with markers every 2 m and posts positioned at both the starting and turning points. Heart rate (HR) and blood pressure were measured using a Comfort M6 blood pressure monitor (Omron, Japan) before the test, immediately after the test, and during the first, second, and third minutes of the recovery period. The changes in HR from baseline to the end of the test (ΔHRpeak) were calculated, along with the changes from baseline to the 1st, 2nd, and 3rd min of recovery (ΔHRrec1, ΔHRrec2, and ΔHRrec3, respectively). The 6MWT was conducted again at the 3-month follow-up. The improvement in walking distance was determined by calculating the difference from the baseline measurements (Δ6MWTD).
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The continuous variables were presented as mean ± standard deviation or as median and interquartile range, whereas the categorical variables were presented as numbers and percentages. The distribution of continuous data was assessed using the Kolmogorov-Smirnov one-sample test and the Shapiro-Wilk test. Continuous measurements were compared using Student’s t-test or the Mann-Whitney U test as appropriate, and categorical variables were analysed using the χ2 test. For all statistical tests, a p-value of 0.05 was considered significant.
Results
Iron deficiency prevalence
We screened 153 patients with significant AS qualified for the TAVI procedure to assess ID prevalence. A flowchart of the study population is presented in Figure 1. Twenty-four patients from the general population did not consent to participate in the study, 3 cases were excluded due to emergency TAVI, and 14 due to baseline inability to walk 100 m.
Demographic data of 112 patients included for further follow-up are shown in Table I. ID was diagnosed in 66 (59%) patients from this population.
Table I
Demographic and clinical data of the study patients
[i] BMI – body mass index, Hb – haemoglobin, ID – iron deficiency, LVEF – left ventricle ejection fraction, MCH – mean corpuscular haemoglobin, MCHC – mean corpuscular haemoglobin concentration, MCV – mean corpuscular volume, Ret He – reticulocyte haemoglobin content, TIBC – total iron binding capacity.
Six-minute walk test
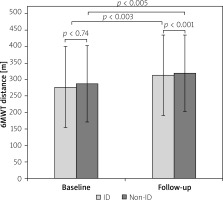
There was no significant difference in the baseline (277.6 ±121.9 m vs. 287.3 ±116.6 m; p = 0.74) and follow-up 6MWT distance (313.1 ±119.6 m vs. 319.4 ±111 m; p = 0.93) between the two study arms (non-ID and ID groups, respectively). It should be noted that in both groups 6MWT distance improved significantly after TAVI compared to baseline results (32.1 ±62.9 m in ID group; p < 0.003 and 39.4 ±68.7m in non-ID group p < 0.005) (Figure 2). For all patients, irrespective of iron levels, the mean change in the 6MWT distance from baseline to 3-month follow-up after TAVI was 35.6 ±65.3 mm. However, 38 (33%) patients failed to improve their 6-minute walk distance during follow-up (25 (37%) from the ID group and 13 (27%) from the non-ID group, p = 0.43). No significant complications were observed during the TAVI procedure that could have influenced the results of the follow-up walk test in the entire study population.
Heart rate recovery
Data regarding HR, ∆HRpeak, and calculations of ∆HRrec, 1∆HRrec, 2∆HRrec, and 3∆HRrec are presented in Table II. There were no statistically significant differences between the two groups of patients.
Table II
Heart rate data at baseline 6MWT
[i] 6MWT – 6-minute walk test, HR – heart rate, ∆HR at peak – difference between baseline HR and HR at the end of 6MWT, 1∆HRrec – difference between baseline HR and HR at the 1st min of recovery, 2∆HRrec – between baseline HR and HR at the 2nd min of recovery, 3∆HRrec – between baseline HR and HR at the 3rd min of recovery.
NYHA class
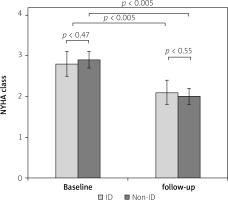
There was no difference in NYHA class between the patients with normal iron levels and the ID group before TAVI (2.9 ±0.3 vs. 2.8 ±0.2; p = 0.47) and at the 3-month follow-up (2.0 ±0.3 vs. 2.1 ±0.1; p = 0.55). Irrespective of iron levels, the NYHA functional class of patients significantly improved (p < 0.05 for both groups) after TAVI (Figure 3).
Discussion
The prevalence of ID in the study population was 59%. Previous analyses have shown that ID is a common comorbidity found in around 50% of patients with stable heart failure [11]. ID prevalence is similar in patients with heart failure with preserved ejection fraction (HFpEF) as it is in those with heart failure with reduced ejection fraction (HFrEF) [12, 13]. ID is the leading cause of anaemia in HF patients [14, 15]. Moreover, it is present in around 46% of anaemia-free individuals with stable systolic HF [11]. Our study showed that the AS patient population eligible for TAVI has a similar proportion of patients with ID as the HF population, and this result is comparable to those obtained in other analyses [16]. Mean haemoglobin levels in the ID group combined with normal MCV but decreased serum ferritin and TIBC indicate the occurrence of non-anaemic iron deficiency in the ID group. Measurement of Ret He in peripheral blood samples, which provides useful information for the diagnosis of an iron-deficient state, showed significantly lower values in the ID group.
Previous studies have indicated that even minor changes in the 6MWT can have significant clinical implications. The minimal clinically important difference in 6MWT among patients with HFrEF and ID was only 14 m at 3-month follow-up [17]. Other studies showed that in patients with HF, minimal clinically important differences ranged from 22 to 90 m [18, 19]. Our results showed no significant differences between the two groups in the baseline and follow-up 6MWT distance. At the same time, both groups showed an improvement in 6MWT distance after TAVI compared to baseline. The improvement in the 6MWT result translated into an improvement in their NYHA classification in both groups. This observation confirms that even a small or moderate increase in 6MWT distance can be clinically significant also in the group of AS patients, irrespective of presence of iron deficiency.
Our results on the differences in 6MWT distance between the two groups, especially after TAVI, may seem surprising. One might have expected that the physical performance of patients with AS and ID might be lower compared to patients with normal iron levels, especially after aortic valve implantation, when the effect of significant AS on exercise capacity was eliminated and could not mask the differences between the two groups. After the procedure, however, the 6MWT result did not differ between the two groups of patients. It should be noted that, although somewhat surprising, these results are similar to previously published studies where there was also no relationship between iron status and physical performance in patients with significant AS. This observation is in contrast to the population of patients with HF, in whom iron deficiency significantly negatively affected exercise capacity [16]. It should be noted that there are only limited data in the literature showing that in patients with severe aortic stenosis, ID is associated with an unfavourable clinical profile and adverse outcomes [20].
Furthermore, the randomized IIISAS trial, which aimed to evaluate the impact of intravenous iron supplementation in the TAVI population, did not reveal any enhancement in clinical status and physical function, nor the quality of life compared to the placebo after a 3-month treatment period [21]. The above observations noted in patients with significant aortic valve stenosis again differ from those in patients with HFrEF. Analysis of the CONFIRM-HF and FAIR-HF randomized controlled trials revealed that HF patients who received iron supplementation demonstrated a notably greater improvement in 6MWT distance compared to those receiving placebo [17].
An impaired chronotropic response to exercise has been identified as a predictor of adverse cardiac events in patients diagnosed with coronary artery disease, heart failure, or pulmonary hypertension confirmed by right heart catheterization [22–26]. Based on our previous findings, we have observed that an adequate chronotropic response during the recovery in pre-TAVI 6MWT could potentially predict enhanced exercise capacity in patients following TAVI during the subsequent 3-month follow-up period [27].
With these observations in mind, the present study analysed the effect of ID on HRR time after the 6MWT. Although ID might have been expected to significantly prolong the time for HR to return to baseline values, no such effect was observed. The group of patients with iron deficiency had only a statistically insignificant prolongation of 1∆HRrec, 2∆HRrec, and 3∆HRrec compared to the group without ID. It is worth noting that this parameter may be at least partially influenced by pharmacotherapy, as the majority of the patients in both groups were receiving β-blockers.
According to ESC guidelines, all ID patients were offered intravenous iron therapy in the outpatient clinic after the study period. However, in light of our results and previously published studies [16, 21], further analysis is required to assess whether AS patients reap the same benefits from iron supplementation as the HF population.
The study has important limitations. Firstly, despite being a prospective observational study, the analysis included only a small number of patients. Additionally, the absence of independent monitoring of the data, including relying on self-reported 6MWT results, can be seen as a significant limitation. Therefore, our results should only be considered as hypothesis-generating. As we did not perform ultrasound or ankle brachial index screening during selection for TAVI, peripheral artery disease might have had some impact on the 6MWT results. However, intermittent claudication was not a cause of physical capacity limitation in any patient. Some recent publications highlight the impact of mental status and COPD on TAVI outcome [28, 29]. Unfortunately, our patients did not undergo mental status assessment. Indeed a few of them suffered from COPD but the presented clinical symptoms were mild and did not impact physical capacity in those patients. Finally, the study did not analyse the influence of different frailty markers on the results obtained.
Conclusions
The results of this study indicate that ID in patients with severe AS has no significant effect on 6MWT results either before or after the TAVI procedure. In this patient group, the multidimensional state of frailty, very often associated with ID, seems to be more associated with adverse outcomes than ID itself [30]. When analysing the presented data, one can question whether the definition of ID used in patients with HF is valid in patients with severe AS. Previous studies, together with our results, may suggest that research is warranted to determine optimal cut-off values for ID in patients with severe AS.