Introduction
Primary/idiopathic hypertrophic osteoarthropathy (PHOA, pachydermoperiostosis) is very rare in children. There are two peaks of incidence of disease symptoms – around 1 year of life and around 15 years of life. The chronic course of the disease is characterized by dynamic development of symptoms in childhood, with the relief or resolution of symptoms in adulthood. The disease is observed mainly in men (7-9 : 1 in relation to women) and more often in the black population. Family occurrence is characteristic. The life expectancy of patients with primary hypertrophic osteoarthropathy is as in the general population [1, 2].
Hypertrophic osteoarthropathy is characterized by a triad of symptoms. These are:
Periosteal bone formation (periostosis) most often of the distal parts of the shafts of long bones (mainly tibia and fibula, followed by phalanges), less often epiphyses. Periosteal bone formation may be accompanied by acroosteolysis [3-5].
Skin hypertrophy (pachydermia). Thickening of facial features, up to the formation of characteristic folds of the hairy scalp and forehead (the so-called lion’s head); hand and foot skin hypertrophy.
Digital clubbing (Hippocratic/Hippocrates fingers, drumstick fingers). It is a characteristic thickening of the soft tissues of the distal phalanges with deformation and matting of nails (watch-crystal nails).
In the “onset” phase, clubbed fingers can be easily seen on physical examination; they arouse patient anxiety. In the development phase of this pathology, measurements of distal phalanges and tests are helpful in the evaluation.
The typical feature is the increase in the ratio of the finger circumference in the middle of the distal phalanx to the finger circumference in the area of the distal interphalangeal joint (phalangeal depth ratio).
In the Schamroth test, in the case of healthy patients, the fingers, after applying the dorsal surfaces of the skin in the area of the distal phalanges of the fingers, create a characteristic diamond-shaped gap, which is not visible in the case of a patient with osteoarthropathy.
For measurement description and schemes see [2].
The dominant clinical symptoms in the course of osteoarthropathy in a large percentage of patients (20-40% [6]), especially in the initial stage of the disease, are pain and other features of arthritis, i.e. swelling, reduced mobility and morning stiffness [3, 7-9].
During the diagnosis of primary hypertrophic osteoarthropathy, attention should also be paid to other characteristic pathologies that may occur in patients. These include increased foot and hand size, seborrhoea, hyperhidrosis, ptosis, delayed closure of cranial sutures/fontanelles, presence of intrasutural bones (ossiculae intersuturae, Wormian bones), and patent ductus arteriosus (PDA). In some patients, the hypertrophy of the periosteum “inwards”, i.e. towards the marrow cavities (endoperiostosis), may lead to bone marrow fibrosis and thus impair haematopoiesis up to pancytopenia [9-11].
It should be noted that in many patients the clinical picture of primary hypertrophic osteoarthropathy is incomplete [12]. The sequence in which the characteristic symptoms, mentioned above, appear also varies, which hinders the diagnosis of the disease [11]. We observe people with only clubbed fingers, and on the other hand, patients with haematological disorders suffering from painful deformities of the joints with impaired functionality and disfiguring hypertrophy of the scalp and face. However, the known causes of hypertrophic osteoarthropathy seem to explain well the observed symptoms and significant phenotypic differences found in individual patients.
Mutations in genes related to the metabolism and transport of prostaglandin E2 (PGE2) cause primary/idiopathic hypertrophic osteoarthropathy. Those are the HPGD gene encoding PGE2 metabolising enzyme and the SCLO2A1 gene encoding PGE2 transport membrane protein. These gene mutations cause increased PGE2 in serum. Increased concentrations of this mediator induce the activity of osteoblasts and fibroblasts, which leads to increased production of collagen and other proteins of the extracellular matrix. PGE2 has also been postulated to influence bone tissue by interacting with the nervous regulation of bone homeostasis [13].
Mutations of genes associated with PGE2 metabolism and transport may be transmitted by autosomal recessive or dominant inheritance, but also show incomplete penetrance [7]. Mutations of individual genes seem to be associated with different clinical pictures of the disease. The manifestation of symptoms in early childhood is associated with a mutation in the HPGD gene (PGE2 metabolising enzyme), with disturbances in the cranial sutures. Mutations of the gene for the PGE2 transport membrane protein, i.e. SLCO2A1, lead to the development of more severe hypertrophic osteoarthropathy in late childhood/early adulthood with skin hypertrophy and bone marrow fibrosis. Gastrointestinal complaints in the course of hypertrophic osteoarthropathy also seem to be associated with the latter mutation, including ulcers, polyps, and hypertrophic gastritis [14, 15]. All this explains the family occurrence of the disease and finding different disease phenotypes in individual patients with the primary form of hypertrophic osteoarthropathy.
Indirect evidence for the pathogenic effect of PGE2 on the development of hypertrophic osteoarthropathy has also been obtained during the observation of newborns and infants, who required pharmacological maintenance of patency of the ductus arteriosus with prostaglandin infusions due to a heart defect. There have been reports of children treated with PGE2 infusions in whom transient, i.e. lasting several weeks, features of osteoarthropathy (mainly clubbed fingers), which did not result from the underlying heart disease, were found. On the other hand, the complaints were related to the duration of the prostacyclin infusion and not to the total dose of the drug [16]. The described pathogenesis of the disease, related to the increased PGE2 concentrations, corresponds well with the fact that the history of patent ductus arteriosus is more frequent in children with primary hypertrophic osteoarthropathy.
Elevated concentrations of PGE2 can be detected in the serum and urine of patients with primary/idiopathic hypertrophic osteoarthropathy. Monitoring of PGE2 in urine was proposed as a method to assess treatment effects of, for example, nonsteroidal anti-inflammatory drugs (NSAIDs) on PHOA [11, 14].
Secondary form of hypertrophic osteoarthropathy
Confirmation of the presence of osteoarthropathy features, as listed above, in a patient requires a thorough differential diagnosis, due to the fact that the vast majority of patients with osteoarthropathy are patients with a secondary form of the disease (95-97%). Careful history and additional tests in a child with clinical signs of osteoarthropathy should exclude chronic heart diseases (cyanotic heart defects and blood vessel defects), respiratory system diseases (cystic fibrosis, chronic or recurrent lower respiratory tract infections, interstitial lung diseases), and neoplastic diseases (including lung metastases). The incidence, course and mortality in cases of secondary osteoarthropathy depend on the cause, while treatment of the underlying disease alleviates or completely resolves the symptoms [17]. In children, secondary causes of osteoarthropathy are relatively less common than in adults [18].
In adults the causes of the secondary osteoarthropathy include neoplastic tumours, infections, chronic inflammation, and chronic hypoxia [2]. The typical causes are chest tumours (non-small cell lung cancer, pleural cancer, thymoma, neoplastic metastases) [19], tumours of the haematopoietic system, cyanotic heart defects, blood vessel defects, vasculitis (including polyarteritis nodosa), endocarditis, respiratory tract defects, pulmonary fibrosis, inflammatory bowel disease [20], cirrhosis, congenital anaemia (mainly thalassaemia), thyroid disease (mainly Graves’ disease), medications (including voriconazole, retinoids), and venous insufficiency of the lower extremities [18-21]. In practice, in adults, osteoarthropathy is in most cases caused by pathology of the respiratory system (90%). Due to its high correlation with thoracic tumours (4-17% of patients with non-small cell lung cancer), it can be considered a paraneoplastic symptom which occurs even several months before the diagnosis of the underlying disease [22].
For a detailed description of primary and secondary hypertrophic osteoarthropathy see [2].
Case description
We present the clinical picture of primary hypertrophic osteoarthropathy in five children, including two pairs of siblings, who were examined in the Paediatric Rheumatology Department (National Institute of Geriatrics, Rheumatology and Rehabilitation, NIGRIR, in Warsaw, Poland) due to pain and features of arthritis. In the course of the diagnosis of joint ailments, patients were diagnosed with primary hypertrophic osteoarthropathy based on the clinical picture and following a complete diagnosis of possible secondary causes of the disease. All patients were referred for genetic diagnosis (test results were not available until the publication of this description). PGE2 levels in serum and urine were not determined.
The detailed characteristics of clinical symptoms and test results are presented in Table 1.
Table 1
Primary osteoarthropathy
The reason for the admission of all five described children to the Paediatric Rheumatology Department was pain and swelling of the joints the patients had experienced from the first years of life (i.e. from the age of 2 years). Their diagnostics was started between the ages of 2 and 7 years.
The complaints concerned knee joints in all patients, in 3 out of 5 patients additionally ankle joints, and in one the wrists. Physical examination revealed swelling or widening of the outlines of the knee and ankle joints in all patients and of the elbows in 2 of 5 cases. Limited joint mobility was found in the knee joints in 3 children, in the elbows in 2 children, and in isolated cases in the hip and wrist joints. Local atrophy of the quadriceps muscles of the thighs was observed in 2 children (Fig. 1).
Fig. 1
Osteoarthropathy. Joint distortion/enlargement of joint contour with swelling of periarticular soft tissues with contracture of the knee joints and partial atrophy of the thigh muscles in a female patient (patient No. 1) with primary hypertrophic osteoarthropathy, with juvenile idiopathic arthritis (JIA) diagnosed and unsuccessfully treated in the past
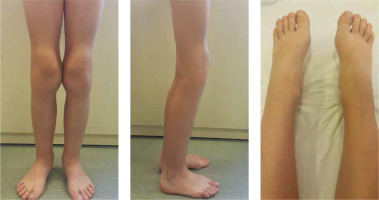
Physical examination revealed clubbed fingers and toes – a symptom which, according to the history of all children, appeared in the first months of life. Only in 2 cases was thickening of the skin of the soles of the feet observed.
The interview showed that in early childhood, 2 children had wide cranial sutures. Radiological examinations revealed disturbances in the mineralization of the skull bones, dilatation and uneven outline of the cranial sutures, in 1 case with visible additional (Wormian) bones. Other changes typical of osteoarthropathy include: hyperhidrosis described in 4 of our patients, seborrhoeic lesions in 2 cases, and large hands and feet in 3 cases. There was no typical scalp hypertrophy.
All patients had normal markers of inflammation. The blood counts were normal in 4 patients, and in 1 girl (patient No. 1) mild microcytic anaemia occurred periodically. No patient was found to have rheumatoid factor or the HLA-B27 antigen. In 2 cases, the presence of antinuclear antibodies (ANA) was found (patient No. 5. 1 : 160 anti-Golgi antibodies, speckled ANA pattern; patient No. 1. 1 : 320 with nucleolus ANA pattern, without the presence of antibodies specific for systemic connective tissue diseases). In the course of rheumatological diagnostics, Borrelia burgdorferi, Yersinia, viral hepatitis and tuberculosis infections were excluded.
Radiological examinations in 2 patients showed typical features of periosteal bone formation, which were visualized in the shafts of the phalanges, radial, ulna, tibia, and fibula (in one of the patients – patient No. 4 – inward bending of the fibulae was also described). Additionally, in one of these patients (patient No. 4) hypertrophy of the right tarsal, heel and ankle bones was described. In 2 patients radiological imaging revealed acro-osteolytic lesions of the bones of the feet. Ultrasound imaging in 2 patients showed inflammatory changes in the synovial membrane: thickening/hyperplasia, but no signs of hyperaemia. Other lesions described in the ultrasound imaging of the joints in individual cases include tenosynovitis, thickening of the subcutaneous tissue and inflammation of Hoffa’s fat pad.
All patients were treated with NSAIDs, which alleviated the pain but did not completely resolve any complaints or symptoms. In 2 cases, intraarticular glucocorticosteroids were used, without any evident improvement. In one of the patients (patient No. 1) with primary hypertrophic osteoarthropathy and previous diagnosis of juvenile idiopathic arthritis treated with methotrexate and sulfasalazine, the diagnosis was revised. Treatment was discontinued without worsening of symptoms.
Discussion
Primary hypertrophic osteoarthropathy is relatively simple to diagnose, when the characteristic picture of the disease is complete. However, the description of paediatric cases from the Paediatric Rheumatology Department presented in this study confirms the difficulties with diagnosing children with primary hypertrophic osteoarthropathy described in the literature, which is related to the incomplete clinical picture of the disease and the dominant osteoarticular complaints at clinical evaluation [10, 11].
Only one patient (patient No. 3) had all the typical symptoms from the characteristic triad of primary hypertrophic osteoarthropathy, i.e. digital clubbing, skin hypertrophy and periosteal bone formation in the bone shaft in X-ray examinations. Moreover, in this child other symptoms of the disease were observed, such as seborrhoea, hyperhidrosis, and disorders of the fontanelle and cranial suture closure. In another boy (patient No. 5) except for the digital clubbing, only skin hypertrophy was found as regards the classic triad of symptoms. In all patients, digital clubbing was accompanied by at least one characteristic “additional feature” of the disease, i.e. hyperhidrosis (in 4 out of 5), seborrhoea (in 2 out of 5), or abnormal fontanelle/cranial suture closure (in 2 out of 5). In 4 out of 5 (2 pairs of siblings) described children, we found a family history of the disease.
Importantly, from the point of view of rheumatological diagnostics, despite the presence of the first symptoms of the disease, i.e. clubbed fingers from birth or the first months of life, and other, less typical symptoms of the disease, the described patients were not diagnosed for these reasons until the onset of symptoms of the locomotor system disorders. This increase in joint pain and swelling eventually led to the diagnosis of primary hypertrophic osteoarthropathy. Joint complaints occurred in all the described patients from the first years of life (i.e. from 2 years of age). Rheumatological diagnosis was often initiated after several years of symptom occurrence. Clinical features of arthritis were found in 3 out of 5 children: joint swelling with limitation of joint mobility. In the remaining two patients, only one of these features, i.e. swelling or limited mobility, was observed. In two patients, the features of thickening/overgrowth of the synovial membrane were confirmed by imaging.
It is known that the dominant clinical symptoms in the course of osteoarthropathy in a large percentage of patients (20-40% [6]), especially in the initial stage of the disease, are pain and other features of arthritis, i.e. swelling, limited mobility and morning stiffness [3, 7-9]. Joint complaints most often occur in symmetrical joints, especially knee joints, ankle joints, wrist joints and finger joints [23]. The most characteristic are joint cavity effusions found on physical examination and imaging examinations [1, 4, 6, 18, 23]. The synovial fluid shows features of non-specific inflammation [11].
It is known that the features of arthritis found in patients, as often the first and dominant symptom of hypertrophic osteoarthropathy, may lead to erroneous diagnosis of systemic connective tissue diseases [7]. There have been reports of patients in whom primary or secondary hypertrophic osteoarthropathy was finally confirmed, and who had previously diagnosed and unsuccessfully treated systemic connective tissue disease due to the features of arthritis – among others, reactive arthritis, rheumatoid arthritis, ankylosing spondylitis, and systemic lupus erythematosus [3, 22].
For the same reason, hypertrophic osteoarthropathy can mimic the symptoms of juvenile idiopathic arthritis (JIA) [18] and thus lead to unnecessary treatment, including immunosuppressive therapy. There are reports in the literature of at least 5 cases of children whose diagnosis of JIA was revised and treatment was discontinued, including immunosuppressive therapy, due to the diagnosis of primary hypertrophic osteoarthropathy [6, 11, 18]. In their paper, Giancane et al. [11] presented patients who were given long-term JIA treatment (NSAIDs, synovectomy, intra-articular steroid administration, immunosuppressive treatment with sulfasalazine and methotrexate, and in one case also tumor necrosis factor (TNF) antagonists – etanercept and adalimumab) and whose JIA diagnosis was withdrawn due to the occurrence of further typical symptoms of the underlying disease, confirmed by genetic testing. Side effects of NSAID therapy administered for misdiagnosed JIA in a patient with primary hypertrophic osteoarthropathy have also been reported [18].
One of the patients treated in our department (patient No. 1), due to the features of arthritis, was diagnosed with JIA in the past and was unsuccessfully treated for several years, initially with methotrexate, then with methotrexate in combination with sulfasalazine. After analysis at the Paediatric Rheumatology Department (NIGRIR), primary hypertrophic osteoarthropathy was confirmed. We discontinued DMARD treatment with no exacerbation of symptoms or progression of pathology in the affected joints observed so far. The patient has persistent joint deformity with swelling of the periarticular tissues, contracture of the knee joints, and partial atrophy of the quadriceps muscles of the thighs (Fig. 1). Imaging studies showed no periosteal bone formation or erosions.
It should be noted that despite joint complaints and significant articular lesions in the physical examination (as in patient No. 1, Fig. 1), in all the described patients, only in 2 patients (patients No. 3 and 4) was typical periosteal bone formation of bone shafts found in radiographic examinations. It is known that the presence of periosteal lesions and their severity (i.e. the thickness of the cortical layer) correlate with the duration of the disease [21], which may be of importance in this case. On the other hand, if imaging tests reveal thickening of the periosteum of long bone shafts in children, causes other than primary hypertrophic osteoarthropathy should always be considered, e.g. child abuse syndrome and very rare genetic syndromes (e.g. Caffey disease) [24].
The use of NSAIDs in the treatment of primary hypertrophic osteoarthropathy is consistent with the known pathogenesis of the disease, in which the cause of the symptoms is an increased concentration of PGE2, as described in the introduction to this paper. In all the described children, we used non-steroidal anti-inflammatory drugs (NSAIDs) and recommended intensive rehabilitation, which resulted in a significant reduction of the pain and improved quality of life. Treatment with NSAIDs most often brings about clinical improvement, but not cure [7, 11, 18, 25]. According to a systematic review of the efficacy of NSAID use in primary hypertrophic osteoarthropathy [25], approximately 70% of NSAID-treated patients (group of 39) showed a reduction in symptoms, mainly pain and arthritis. Other treatments for primary hypertrophic osteoarthropathy include intra-articular glucocorticosteroid injections [18] (which we used in two patients with transient improvement (patients No. 3 and 4), as well as bisphosphonates [8]. Surgical treatment is used in patients with skin hypertrophy and ptosis.
Summing up, the described histories of the patients of our department confirm the need to consider primary hypertrophic osteoarthropathy in the differential diagnosis of joint pain and inflammation in children. This allows one to avoid the use of unnecessary intensive JIA treatment, including immunosuppressive treatment, in a disease with a different pathogenesis and a good prognosis [18]. Giancane et al. [11] recommend that in patients with swollen and painful joints, with a history of PDA, delayed closure of cranial sutures or fontanelles, with Wormian bones, primary hypertrophic osteoarthropathy should be taken into account. In such a situation, the patient may not yet present the classic symptoms of osteoarthropathy. In addition, the diagnosis of primary hypertrophic osteoarthropathy should be considered in a patient diagnosed with juvenile idiopathic arthritis with no expected improvement observed with the treatment with disease-modifying drugs, but who improves with NSAIDs [11].
In adults, primary or secondary hypertrophic osteoarthropathy should be considered in the diagnosis of arthritis in a patient with a chronic disease and digital clubbing [18]. It is also necessary to consider the cause of the more frequent secondary form of the disease in adults as compared to the paediatric population.
Conclusions
Primary hypertrophic osteoarthropathy is a very rare disease in developmental age; its slow course, incomplete clinical picture and the presence of predominant joint symptoms may result in the incorrect diagnosis of inflammatory arthropathy and the use of ineffective therapy. The present description of five cases of children from the Paediatric Rheumatology Department indicates diagnostic difficulties in children with primary hypertrophic osteoarthropathy. All of them were examined because of pain and features of arthritis. We observed an incomplete clinical picture of the disease. One patient required a revision of the previous diagnosis of JIA and discontinuation of ineffective treatment with disease-modifying drugs (DMARDs). For these reasons, primary hypertrophic osteoarthropathy should always be considered in the differential diagnosis of inflammatory arthritis, especially juvenile idiopathic arthritis.


