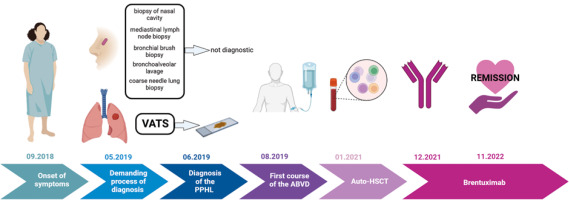
Introduction
Lung involvement in Hodgkin’s lymphoma (HL) occurs in approximately 15–40% of HL cases [1]. However, primary pulmonary Hodgkin’s lymphoma (PPHL) is an exceptionally rare diagnosis, with fewer than a hundred cases reported between 1927 and 2006. Primary pulmonary Hodgkin lymphoma represents less than 0.5% of primary lung malignancies and less than 1% of all pulmonary lymphomas [1–4]. Primary pulmonary Hodgkin lymphoma is defined as histologically confirmed HL localized in the lung, with or without hilar or mediastinal involvement [5, 6]. Due to its rarity and nonspecific symptoms, PPHL is rarely considered in the initial differential diagnosis of lung diseases. In this case report, we present the perplexing and occult presentation of PPHL in a 53-year-old patient, which posed numerous challenges in terms of diagnosis and treatment.
Case report
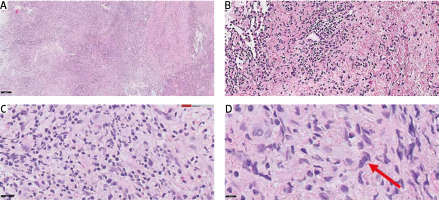
A 53-year-old woman was admitted to the hospital with weakness, weight loss, dry cough, and dyspnoea that had been progressively worsening for a few weeks. Clinical examination revealed nasal cavity ulceration. Blood tests showed elevated levels of C-reactive protein (CRP) (33.2 mg/l) and white blood cells (21.5 × 103/µl) with neutrophilia (17.8 × 103/µl) and lymphopaenia. Radiological findings on computed tomography (CT) revealed infiltrative changes within the third segment and lingula of the left lung, along with disseminated nodular changes. The presence of a lung mass raised suspicion of carcinoma, leading to several diagnostic procedures, including bronchial brush biopsy, mediastinal lymph node biopsy, and bronchoalveolar lavage. However, none of them showed the presence of atypical cells. Nasal cavity ulceration, elevated CRP, and lung infiltrative changes led to suspicion of granulomatosis with polyangiitis (GPA). Therefore, biopsies of the nasal cavity and lung were performed. However, both tests yielded ambiguous results with equivocal features of GPA. Despite not having a definitive diagnosis of granulomatosis associated with polyangiitis, it was decided to initiate a course of steroids (60 mg of Encorton per day). However, the therapy did not result in clinical improvement, and the patient developed a low-grade fever and an itchy erythematous rash. Moreover, lung lesions demonstrated radiological progression, prompting a repeat diagnostic evaluation of the pulmonary changes. Video-assisted thoracoscopic surgery was performed. Histopathological analysis showed lung tissue with disrupted histoarchitecture, fibrosis, and lymphoid infiltration forming nodules, without features of phlegmonous inflammation or granulomatous vasculitis, but with the presence of dispersed larger cells resembling Hodgkin’s and Reed- Sternberg-like cells (HR-S) (Fig. 1).
Fig. 1
Video-assisted thoracic surgery acquired samples of left lung lesion, haematoxylin and eosin staining: 50× magnification (A), 100× magnification (B), 200× magnification (C), 400× magnification (D) (red arrow indicates the suspected cell with [Hodgkin’s and Reed-Sternberg-like] features)
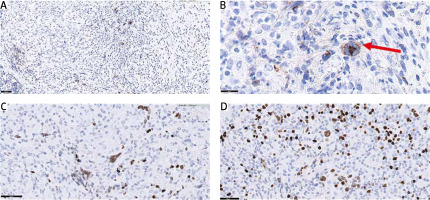
A complex immunophenotyping was performed to differentiate PPHL from other non-Hodgkin B-cell lymphomas, particularly lymphomatoid granulomatosis (Fig. 2). Immunohistochemistry revealed negativity for CD20, (octamer binding protein 2) OCT-2, and the Epstein-Barr virus antigen, weak positivity for (B cell lineage specific activator protein) PAX5-BSAP, and a background with a predominance of CD3-positive T-cells. These findings excluded a diagnosis of non-Hodgkin’s B-cell lymphoma. Considering that the primary lesion was located in the upper lobe of the left lung, the diagnosis of PPHL was made.
Fig. 2
Immunophenotyping of the Hodgkin’s and Reed-Sternberg-like (HR-S) cells. Immunohistochemical staining of CD30 showing positivity in HR-S cell (50× and 100× magnification, respectively). Hodgkin’s and Reed-Sternberg-like cell indicated by a red arrow (A, B), immunohistochemical staining of B cell lineage specific activator protein (PAX5-BSAP) showing weak positivity (C), immunohistochemical staining of octamer binding protein 2 (OCT-2) showing negativity in HR-S cells (D)
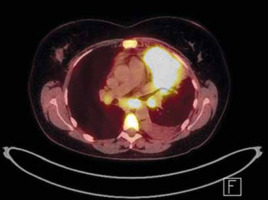
Next, a bone marrow biopsy and positron emission tomography (PET) scan were performed (Fig. 3). The disease was classified as stage IIIB according to the Ann Arbor pulmonary lymphoma staging system, which includes symptoms and the location of the disease, based on PET imaging demonstrating lymph node involvement on both sides of the diaphragm and the patient experiencing fever and weight loss [7].
Fig. 3
Positron emission tomography imaging of the chest (August 2019). In segment 1/2 and in segment 3 and 4, metabolically active tumour (84 × 52 × 64 mm SUVmax = 19.9) with visible small satellite nodules up to 10 mm (SUVmax up to 4.9), enlarged, metabolically active lymph nodes of both lung hilum (20 × 16 mm SUVmax = 10.8 – on the left side, and 16 × 12 mm SUVmax up to 5.8 – on the right side), enlarged, metabolically active subacute lymph node size (25 × 20 mm, SUVmax = 11.8)
Following the final diagnosis of PPHL, the first-line treatment for classical Hodgkin’s lymphoma, the Adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) regimen, was administered [8, 9]. Unfortunately, after this therapy, PET imaging revealed progression of the lesion in the left lung and involvement of retroperitoneal lymph nodes. In response to disease progression, intensified treatment with the BEACOPP escalated regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone in escalated doses) was initiated. Three cycles of this regimen were completed, but it also proved ineffective as PET imaging showed further progression of the lesion in the left lung.
Subsequently, high-dose chemotherapy for HL, specifically the ICE regimen (fractionated ifosfamide, carboplatin, etoposide), was administered. However, after 2 cycles of this regimen, PET imaging once again showed progression of the previous lesion in the left lung, as well as new metabolically active lymph nodes in the left hilum and metabolically active bone marrow. Consequently, the patient was treated with the dexaBEAM regimen (dexamethasone, carmustine, etoposide, cytarabine, and melphalan), which had previously been proposed as a salvage therapy for Hodgkin’s disease [10]. However, after 2 cycles of dexaBEAM, PET imaging revealed new lesions in the right lung (segments 3 and 6) and left lung (segment 6). In light of the disease progression, the patient underwent the IGEV regimen (ifosfamide, gemcitabine, and vinorelbine) for mobilization and collection of CD34+ cells for autologous stem cell transplant (auto-SCT). After the mobilization, the patient received 2 cycles of BDG (bendamustine, gemcitabine, and dexamethasone). Positron emission tomography imaging showed a response with a Deauville score of 3. The auto-SCT was performed in January 2021.
However, the period of drug-induced aplasia was complicated by febrile neutropaenia, bacteraemia, gastrointestinal mucositis, as well as infection by Clostridium difficile and Candida glabrata. Additionally, slow regeneration of platelet parameters was observed, and a fine-needle bone marrow aspiration biopsy revealed a few megakaryocytes with poor platelet cleavage.
Due to the disease’s resistance to multiple treatment lines and the increased risk of relapse or progression after auto-SCT, the patient became eligible for treatment with brentuximab vedotin (BV) through the Ministry of Health’s drug reimbursement program for HL patients (B.77). Currently, the patient has achieved a partial response after the 16th cycle of BV. The treatment is well tolerated, with only grade 1 peripheral neuropathy reported as of June 2023.
Discussion
Primary pulmonary lymphomas are rare, accounting for less than 1% of lung cancers, with fewer than 100 reported cases [5]. Incidence rates have been slightly higher in women (M:F ratio 1:1.4), with age ranging between 12 and 82 years (mean 42.5 years) [4]. The most common symptoms include cough, dyspnoea, weight loss, fever, and night sweats [11]. However, in our case, the patient presented with a misleading clinical picture resembling granulomatosis with polyangiitis, which, to our knowledge, has not been previously reported in PPHL cases. This is particularly interesting because GPA has been reported primarily in diffuse large B-cell lymphoma and lymphoid granulomatosis patients [12, 13]. In our case, both diagnoses were considered in the differential diagnosis but were ultimately excluded based on comprehensive histological and immunophenotypic studies.
Typically, PPHL presents in the superior portions of the lungs, in contrast to secondary pulmonary Hodgkin lymphoma, which can involve any region of the lungs [2]. Despite the typical localization in our patient (left lung with disseminated lesions in the upper lobes of both lungs), 4 biopsies were necessary to confirm PPHL, highlighting the importance of accurate lung biopsy supported by high-quality radiographic imaging and consultation with a pulmonologist. Histopathologically, the patient exhibited nodular sclerosis Hodgkin’s lymphoma, which is the most common subtype of PPHL [14].
Most HL patients respond well to chemotherapy; however, 5–10% will exhibit resistance to initial treatment, and 10–30% will experience relapse [15]. Until recently, cytotoxic chemotherapy has been the sole approach used to achieve a complete response (CR) before undergoing auto-SCT for relapsed or refractory HL patients. The salvage chemotherapy regimens that are often used include ICE and DHAP (cytarabine, cisplatin, dexamethasone). Commonly employed salvage chemotherapy regimens encompass ICE and DHAP, which demonstrate a limited CR rate of approximately 25%, alongside a relatively high objective response rate (ORR) of 88% [16]. Despite other proposed regimens in the event of treatment failure, these chemotherapy protocols remain viable choices for pre-auto-SCT salvage treatment [16]. At present, no specific therapeutic recommendations exist for PPHL, so patients are managed in accordance with HL guidelines. Adverse prognostic indicators for PPHL include advanced age, B symptoms, bilaterality, multiplicity, pleural effusion, and cavitation [16]. Some studies suggest a combination of radiotherapy and chemotherapy, but this approach has been associated with an increased risk of radiation pneumonitis [2, 4]. In the presented case, the patient received 5 different chemotherapeutic regimens: ABVD, BEACOPP, ICE, dexaBEAM, IGEV, followed by auto-SCT and BV. The therapeutic effect was finally achieved after the implementation of BV, providing further confirmation of the Hodgkin’s lymphoma diagnosis. In one of the largest available analyses, Radin reported a two-year disease-free survival in only 15 (39.5%) out of 38 PPHL cases [4]. However, this study included patients who underwent various treatments, including radiation, surgery, or chemotherapy, with the latter primarily being the MOPP regimen (mechlorethamine, vincristine, procarbazine, prednisone), which is currently not recommended. Conversely, recent analyses and individual case reports on ABVD administration are optimistic, demonstrating mostly CR in patients [17, 18]. Given these circumstances, the prognosis of PPHL and the outcomes of novel chemotherapy regimens remain uncertain, necessitating further studies involving a larger patient cohort. In the described case, exceptional chemo-resistance was observed, with a sustained response achieved following BV treatment.
Conclusion
Our case presents an extremely rare pulmonary tumour with a misleading clinical and pathological presentation, a protracted diagnostic process, and high resistance to therapy (Fig. 4). Therefore, this case report contributes to increasing awareness of Hodgkin’s lymphoma in the differential diagnosis of lung diseases.