Introduction
Growth hormone deficiency (GHD) is a medical condition characterised by insufficient hormone secretion, which results in reduced growth velocity and may lead to a short stature. Short stature is defined as height standard deviation score (SDS) less than –2 SD or below the third percentile for age and gender. The prevalence ranges from 1 : 10,000 to 1 : 4,000 worldwide [1]. We can distinguish 3 distinct kinds of GHD based on aetiology: acquired, congenital, and idiopathic [2].
The determination of a diagnosis depends on auxological, radiological, and biochemical testing, including provocation tests with clonidine, arginine, glucagon, insulin, levodopa, and GH-releasing hormone (GHRH) [2]. Growth hormone secretion should be assessed in at least 2 different GH stimulation tests, and results below normal allow a diagnosis of GHD [3].
It is now well recognised that treatment of GHD with recombinant human growth hormone (rhGH) is both safe and effective [4, 5]. According to current research, patients increase their height by up to 8.72 ±2.27 cm during the first year of the therapy (p < 0.01) [6].
However, determining the perfect treatment strategy (e.g. rhGH starting doses, dose adjustments) remains a source of debate among researchers [7, 8]. A considerable number of patients do not achieve satisfactory therapy outcomes. Potential factors influencing the response to rhGH are numerous and include short stature aetiology (e.g. in Turner syndrome higher doses are recommended), genetics (e.g. whole GH1 gene deletions), puberty, body weight and inadequate nutrition, additional diagnoses (e.g. celiac disease and inflammatory bowel disease, skeletal dysplasia), medications that impair growth (e.g. radiation to the spine or growth plates), adherence (breaks in the treatment, following recommendations), and the patient’s psychosocial environment [9].
Aim of the study
With this cohort study, we aim to evaluate the predictive effect of height increase in the first year of rhGH treatment on long-term therapy outcomes among children diagnosed with GHD. We hypothesise that poor primary response may lead to worse rhGH treatment effects.
Material and methods
Study design
A single-centre, retrospective cohort study was conducted using collected medical records regarding short-stature children treated with rhGH in the Department of Clinical Paediatrics, Provincial Specialist Children’s Hospital in Olsztyn in Poland. Inclusion criteria gathered patients diagnosed with isolated GHD (multihormonal hypopituitarism were not included) with at least one year of follow-up. The diagnosis of GHD was carried out according to a standard scheme, using 2 different GH stimulation tests [9, 10]. Patients were enrolled in the study regardless of their pubertal status at the start of rhGH treatment. The exclusion criteria were lack of data (caused, for example, by continuation of treatment in other medical facilities) and the presence of diseases that might affect growth, which makes these findings incomparable.
Outcomes measurement
The following information were collected from the hospital database: patient’s sex, chronological age (CA), bone age (BA), height, weight, insulin-like growth factor 1 (IGF-1) level, and rhGH dose for up to 10 years with one-year intervals.
To measure the effectiveness of rhGH treatment, the height, body mass index (BMI), and IGF-1 were normalised for chronological age and gender, using World Health Organisation (WHO) computation of percentiles and Z-score, and somatic development indices for Polish children [11–13]. The height velocity (HV) SDS was based on the mean change of height SDS in consecutive years from the baseline to the fifth year.
Children were divided into 2 groups according to the change (Δ) in height SDS after the first year of rhGH treatment. The first group (good responders, GR) included patients with a greater than 0.5 height SDS increase. The second group gathered patients who had a lower than 0.5 height SDS increase (poor responders, PR). The 0.5 SDS limit splitting the 2 groups was drawn from current consensus guidelines determining successful first-year response to rhGH treatment, which includes an increase in height SDS of more than 0.3–0.5 and corresponds with studies related to ‘poor responders’ [7, 14].
Statistical analysis
Data were analysed using descriptive statistics. The values of the same parameter of GR and PR groups were compared using Student’s t- test for independent groups or Mann-Whitney U test (parametric data), and the χ2 test (non-parametric data). The correlations between the Δheight SDS in the first year and the Δheight SDS at later stages of observation, and with HV SDS were assessed using Pearson’s correlation coefficient for all patients. A p-value of < 0.05 was considered significant. All calculations were preceded by Shapiro-Wilk and Levene tests. The analysis was performed using Statistica (data analysis software system), version 13, http://statistica.io (accessed on 10 August 2022) TIBCO Software Inc., Krakow, Poland (2017).
Bioethical standards
Ethical approval was gained from the Provincial Specialist Children’s Hospital in Olsztyn Research Ethics Committee (REC reference 29/ZE//2022/WSSD). Our institution gave the appropriate consent to conduct the study. Due to the retrospective nature of the study, consent to participate in the study was not obtained from patients or legal guardians. However, they signed consent to treatment with rhGH.
Results
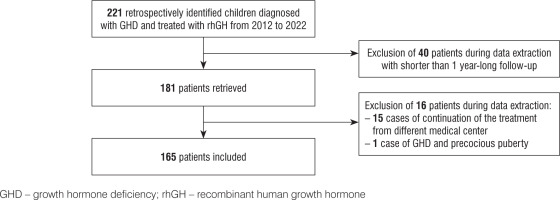
A total of 221 children were diagnosed with GHD and treated with rhGH in the Department of Clinical Paediatrics, Provincial Specialist Children’s Hospital in Olsztyn in Poland between 2012 and 2022. The treatment of short stature was conducted in accordance with the Polish recommendations for rhGH therapy [15]. After screening 221 children, 40 of them were excluded according to the follow-up criteria. The observation period ranged from 1 to 10 years; mean follow-up was 4.32 ±1.80 years. Finally, among 181 retrieved cases, 16 individuals were excluded: 15 patients with a lack of data, caused by continuation of treatment in different medical facilities and one child with GHD and precocious puberty. Finally, 165 children were included in the study. The follow-up rate was 91% (Fig. 1).
At recruitment the mean patients’ CA was 14.03 ± 3.06 years and ranged from 5 to 19 years of age. The GR group consisted of 88 children (53%): 32 girls and 56 boys (36% and 64%, respectively). The PR group consisted of 77 patients (47%), 29 girls and 48 boys (38% and 62%, respectively). At the start of rhGH therapy (baseline) the mean CA was 10.72 ±3.33 years and ranged from 3 to 17 years of age. Mean baseline high SDS was –2.58 ±0.65. There were no statistically significant differences between the patient characteristics (p > 0.05; Table I).
Table I
Characteristics of the groups
Given the small number of children with longer than 5-year follow-up (low certainty of evidence), the authors decided not to comply with these findings in the results. In the GR group the mean Δheight SDS (baseline to 2nd, 3rd, 4th, and 5th year) were 1.29 ±0.40, 1.55 ±0.57, 1.72 ±0.71, 2.04 ±0.50, respectively. In the PR, the mean Δheight SDS were lower at each subsequent stage of observation, reaching 0.60 ±0.35, 0.94 ±0.49, 1.22 ±0.70, 1.14 ±0.49. The common rhGH efficacy comparison factor – the mean HV SDS (based on Δheight SDS in consecutive years between baseline and fifth year) was about twice as high (1.19 ±0.41/year) for the GR group comparing to the PR group (0.59 ±0.38/year). All differences between the groups were statistically significant (p < 0.05; Table II).
Table II
Treatment outcomes
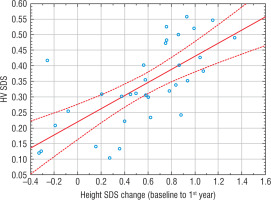
The Pearson’s correlation coefficient showed in all patients a high correlation between the Δheight SDS in the first year and the Δheight SDS at each later stage of observation (baseline to 2nd, 3rd, 4th, and 5th years), and with HV SDS (Table III, Fig. 2).
Table III
Correlations between Δheight SDS (baseline to the first year) and treatment with rhGH outcomes
[i] “Baseline” refers to the start of rhGH treatment. Value adjusted based on the standard scale of Pearson’s correlation coefficient: 0 < r ≤ 0.19, very low correlation; 0.2 ≤ r ≤0.39, low correlation; 0.4 ≤ r ≤0.59, moderate correlation; 0.6 ≤ r ≤0.79, high correlation; 0.8 ≤ r ≤1.0, very high correlation SDS – standard deviation score; HV – height velocity
Discussion
The current study was designed to assess the predictive effect of height increase in the first year of rhGH treatment of GHD children on long-term therapy outcomes. Patients were divided into 2 groups: good responders (GR) and poor responders (PR). Their growth was examined up to 5 years after the onset of the therapy. After 5 years of treatment, the GR’ mean Δheight SDS was 2.04 ±0.50, whereas the PR’ mean Δheight SDS was 1.14 ±0.49. The main finding of our study is that the primary response to the rhGH treatment seems to be a reliable predictor for long-term therapy effectiveness.
Despite years of experience using rhGH to treat GH-deficient children, there is still a disagreement regarding what constitutes a “poor response” [16, 17]. Bakker et al. developed age-, gender-, and diagnosis-dependent curves for HV SDS during rhGH treatment. In this study, it was suggested that patients with a first year HV SDS below –1 SD be classified as “poor responders” [18]. In a different study, Ranke et al. divided GHD patients into 2 groups according to the results of tests for maximum peak GH level (maxGH). The first group, described as ‘severe GHD’, consisted of patients with maxGH less than 5 µg/l. In this cohort, a height SDS increase < 0.4 was considered an inadequate response to rhGH during the first year of the treatment. The second group, described as ‘less severe GHD’ consisted of patients with maxGH in the range of 5–10 µg/l. In this cohort, a height SDS increase < 0.3 was considered an inadequate response to rhGH during the first year [19]. Contradictorily, it is worth mentioning that an increase in height SDS < 0.3 at ages 3 to 8 years is within the range of normal variance of height measures seen over one year [20]. A distinct definition was proposed in a study performed by Bang et al. In which short children with isolated GHD were classified as ‘poor responders’ by the criterion of height gain of SDS < 0.5 [7]. Considering the variety of definitions, we decided to apply in our research the definition proposed by Bang et al., which stands as a compromise in comparison to other studies and is straightforward to implement.
In an effort to focus on our core hypothesis, we attempted to remove or at least recognise the effect of the following factors influencing the pattern of response to rhGH therapy [21]. The rhGH dose, response to the rhGH treatment (expressed in height SDS increase), patients’ adherence, and CA at the start of the therapy are all critical determinants of the final height result, especially in the “poor responders” group [22, 23]. Modifications to the mentioned factors should enhance the long-term success of the treatment.
There have been different approaches to establish safe and sufficient rhGH dosage in GHD therapy [24, 25]. Mauras et al. conducted a randomised trial to examine the effectiveness and safety of regular rhGH therapy dosage (0.3 mg/kg/week) vs. high rhGH therapy dosage (0.7 mg/kg/week) in GH-deficient adolescents who had previously been treated with rhGH for at least 6 months. High dosage rhGH therapy in adolescents significantly increased near-adult height and height SDS, did not accelerate the rate of skeletal maturation, and appears to be well tolerated and safe when compared to traditional treatment [25]. On the contrary, a randomised study by Coelho et al. has shown a lack of significant effect on the final height of children with GH deficiency between the 2 groups. In the mentioned study the first group received a regular rhGH dosage (5 mg/m2/week), and the second group received a high rhGH dosage (10 mg/m2/week). Moreover, the curve for height gain in relation to the dose of rhGH was comparatively flat, even at much higher doses [26, 27]. Additionally, enhanced near-adult height outcomes observed in certain trials may simply be a pharmacological side effect of supraphysiological rhGH treatment rather than the restoration of a deficient hormone [25, 28]. Some endocrinologists administer a set dosage of rhGH; however, the majority employ an auxological-based dosing method. As a rule, this would entail beginning at the lower end of this dosage range and titrating to the higher range, based on the patient’s response to therapy with an assessment of IGF-1 concentrations, to ensure the patient was neither overtreated nor undertreated [29]. In contrast to techniques of rhGH dosage based on weight, Cohen et al. showed that maintaining the IGF-1 level close to the mean for age and gender resulted in a comparable 2-year growth response while utilising a lower mean dose of rhGH [30, 31]. Since population studies have shown a link between greater IGF-1 and various malignancies in the general adult population, this method avoids supraphysiological blood levels of IGF-1, indicating that it may increase the safety of the treatment [32]. Current consensus recommendations by the Paediatric Endocrine Society state that the dosage should fall between 0.16 and 0.24 mg/kg/week, with IGF-1 levels taken into consideration and with individualisation of subsequent dosing [33, 34]. Because our study was conducted in Poland, our patients received the doses of rhGH within the range 0.1–0.33 mg/kg/week in accordance with the Polish Society of Paediatric Endocrinology guidelines, which falls within the presented global consensus range [10]. The rhGH dose protected the patients from over- or under-treatment and prevented outcomes from being disrupted by the rhGH dose.
A first-year height rises of 0.5 SDS converts to an average final height gain of almost 1 SDS in prepubertal GH-treated children with isolated GHD [35]. A model by Kristorm et al. based on the observed first-year growth response on rhGH made valid predictions of up to 7 years of prepubertal growth in children with GHD [36]. Consideration is given to a variety of potential growth response criteria. Several parallels can be seen between our results and the discussed studies. In our study the GR group, which gained over 0.5 SDS in the first year, reached a height gain of 2.04 ±0.50 SDS after 5 years, which is almost 1 SDS higher than the PR group, which reached 1.14 ±0.49. Additionally, the GR group had a mean HV SDS of 0.57 ±0.24/year, whereas the PR group had only 0.29 ±0.20. In our study, the difference in HV SDS [/year] between the GR and PR groups remained relatively comparable, making it a valid predictor of future height growth expectations.
Patients and parents may find that the increase in height SDS, especially at the beginning of the therapy, is the most significant metric of therapy success. It shows how the patient’s height will vary after therapy and shows growth deviation in comparison to peers. These are clinically and psychologically significant factors and can worsen the adherence, which further lowers the treatment effectiveness [37]. Our study shows that, while some patients may have slower height SDS growth, they are still gaining a considerable amount of height SDS every year. We aim to keep the adherence to therapy as high as possible by creating realistic growth expectations.
Low treatment adherence can be a reason for “poor response”. There is a strong correlation between a high adherence rate and an optimal clinical response during the first year and long-term rhGH treatment [38–40]. In the study performed by Munoz et al., patients who had higher adherence rates experienced greater height gains after 2 years of treatment. A decline of 1.8 cm was seen for every 10% drop in adherence. After 2 years of treatment, subjects who received a higher dose of rhGH experienced greater HV [40]. Loftus et al. also showed that on average, adherent patients gained an additional 1.8 cm over one year compared with non-adherent patients, adjusted for covariates. The medication possession ratio (MPR) was used to assess adherence during the study, patients were classified as adherent (MPR ≥ 0.8) or nonadherent (MPR < 0.8) [41]. Bozzola et al. discovered a proficient level of therapy adherence in a group of children utilising an electronic auto-injector device for up to 3 months. A satisfactory level of adherence was defined as injecting at least 92% of doses over the 3-month period. Additionally, in the first month, 75.1% of patients missed no shots, and in the second and third months 66.7% of children missed shots [42]. In a study by Bagnasco et al., it was shown that 24.4% of patients missed one or more injections during a typical week and were thus considered non-adherent. The most frequently reported reasons for missing a dose were being away from home (33.3%), forgetfulness (24.7%), not feeling well (12.9%), and pain (10.3%) [43]. One of our study’s limitations is the lack of precise data on patient adherence. Although patients and their parents were asked about adherence at each recurring visit, it was always self-reported, which has a significant impact on our study.The time of the onset of the therapy can also induce a “poor response”. Standardisation makes it possible to express HV SDS regardless of age and gender [7]. Increases in height SDS and HV SDS during the first year of rhGH treatment in multiple diagnoses are highly age-dependent [19, 44, 45]. The physiology of normal, linear growth provides a significant explanation for this age dependency. The number of years of pre-pubertal rhGH treatment predicts final height, with studies reporting better outcomes when treatment is initiated at a young age [46]. Munoz et al. showed that an average height increase of 2.5 cm was linked to beginning the treatment before the onset of puberty [40]. It is worth mentioning that serum IGF-1 levels change with age. Unfortunately, IGF-1 serum concentration, especially in prepubertal children, coincides with the IGF-1 serum range identified in GHD, which results in difficulties with therapy optimisation [11]. Although the bone age-adjusted and puberty stage-adjusted IGF-1 concentrations would be normal, they also rise sharply throughout puberty. As a result, in a child with delayed puberty and development, the IGF-1 concentration might seem to decrease [29]. Considering how the starting age of the rhGH therapy could impact our study, we took care in the comparison of the mean age of our participants. In the GR group clinical and bone age were, respectively, 10.63 ±3.67 and 9.63 ±3.50 years, while in the PR group clinical and bone age were, respectively,10.82 ±2.91 and 10.12 ±9.16 years. In both cases differences between the groups were statistically insignificant and equal to p = 0.905 for the clinical age and p = 0.979 for the bone age.
The effectiveness of rhGH therapy should be evaluated after 12 months of treatment. All the factors listed above imply that specialists performing rhGH treatment should take additional steps when a patient after the first year of treatment is qualified as a “poor responder”. Guidelines for identifying and treating patients who do not respond well to rhGH treatment have already been suggested. The core structure of these guidelines strongly correlates with the factors mentioned in previous paragraphs [22]. Recognising poor or inadequate responses will lead to more successful short-stature management, as well as awareness of the most appropriate therapeutic strategy.
There are 2 major limitations in the study. The main issue is the non-optimal number of parameters included throughout the investigation. When researching the predicted implications of various characteristics, more data may typically assist in producing better findings. Another constraint was the limited number of patients. The total number of patients in our research was 165. In comparison to research conducted on large datasets, our study may be less credible [47, 48].
Conclusions
In conclusion, the primary response to the rhGH treatment in GHD children seems to be a reliable predictor for long-term therapy effectiveness. Implementation of long-term follow-up is essential for the early detection of patients who are more likely to fail and for the establishment of additional therapeutic strategies. However, future research is needed, especially evaluating factors causing a poor primary response, including genetics and epigenetics.

 POLSKI
POLSKI