Introduction
Prolonged and unscrupulous antibiotic usage, which is rampant in resource-limited settings of Asia and Africa [1–3], continues to exacerbate the burden of antibiotic-associated diarrhea and, by extension, Clostridioides difficile infection (CDI). Antibiotics suppress protective gut microbiota, conferring a growth advantage to C. difficile and other pathogenic bacteria. The organism produces toxins A and B, which are cytotoxic to intestinal cells and downregulate the expression of NHE3 and DRA [4, 5]. Damaged epithelial cells fail to perform their physiologic functions. Clinical signs can be traced to this mechanism, as well as collateral provocation of immune cells. Assessment of disease severity is based on the number of bowel movements, the color of stool, white blood cell count, renal function test, and inflammatory markers, and guides the aggressiveness of management. Additional risk factors include immunosuppressive situations such as HIV/AIDS, age above 65 years, diabetes, and chronic kidney disease, among many others [6–8].
Clinical guidelines typically recommend vancomycin or fidaxomicin as first-line treatments for CDI, reserving fecal microbiota transplant (FMT), and bezlotoxumab for recurrent cases [9–11]. However, antibiotic use in CDI treatment can disrupt beneficial microbiota, leading to high recurrence rates. FMT aims to restore gut microbiota depleted by antibiotics or gut pathology, potentially inhibiting C. difficile proliferation [12]. Despite its efficacy, FMT faces challenges such as donor recruitment and lack of standardization [13, 14]. Correlational studies have associated FMT with adverse effects such as thrombocytopenic purpura, peripheral neuropathy, and autoimmune diseases. In contrast, probiotics containing Lactobacillus, Bifidobacterium, and Saccharomyces strains offer a simpler approach as they can be derived from natural foodstuffs or commercially prepared and packaged as dietary supplements or capsules. Probiotics compete with pathogens for nutrients, produce antimicrobial peptides, and strengthen the gut barrier, reducing opportunistic infections and promoting gut health [15].
Despite the stellar mechanisms propounded above, the clinical efficacy of probiotics is heterogeneous, heavily influenced by strain characteristics and target population. Across the literature, however, most of the strains have exhibited beneficial effects on antibiotic-associated diarrhea (AAD). When offered as adjunctive therapy for CDI, probiotics promote faster degradation of toxins A and B produced by C. difficile, besides suppressing gene-related synthetic mechanisms. They further antagonize gut colonization and inflammatory mediators induced by C. difficile [16, 17]. These mechanisms, coupled with ease of administration and low cost, provide an appealing method of countering CDI. Therefore, we aimed to evaluate the evidence for probiotic use in the treatment and prevention of AAD in adults.
Methodology
Study design
This meta-analysis combined various eligible randomized controlled clinical trials (RCTs) that studied the effect of probiotics on AAD and CDI due to conflicting results from a single RCT on the benefits of probiotics.
Inclusion and exclusion criteria
Only articles published in English between January 2010 and October 2023 were considered for inclusion in this review. The selected studies focused on human participants aged 18 years and above who were diagnosed with diarrhea, regardless of whether they were in hospital or nonhospital settings. The primary objective of these studies was to examine the efficacy or effectiveness of probiotic use compared to either a placebo or alternative treatment. Probiotics could have been administered concurrently with antibiotics or before/after their use. The comparator group either received a placebo or no intervention. The main outcomes of interest were the incidence of AAD and CDI, with some studies also reporting secondary outcomes such as the severity and duration of CDI. Non-randomized studies (e.g., case reports, observational studies) and articles lacking full texts were excluded. Additionally, studies involving pediatric populations or both pediatric and adult populations were not considered for inclusion.
Information sources
EMBASE, Medline via PubMed, Scopus, and Google Scholar were searched for eligible articles. Most RCTs registered with clinicaltrials.gov or the WHO International Clinical Trials Registry were published and indexed. Hand-searching bibliography lists from review articles also retrieved some eligible studies. Grey literature was not searched due to potential duplication of information.
Search strategy
The initial search terms that were used to pilot the study included the following terms: “probiotics”, “AAD”, and “adults”. Using MeSH and Emtree terms, and synonyms, the strategy was expanded and implemented as per the protocol of various databases.
Study selection
The authors screened titles and abstracts using a pre-specified eligibility criterion. A screening form with scores (No/Yes/Maybe) was used: “No” for exclusion, “Maybe” for unclear titles/abstracts, and “Yes” for full-text review. The JBI standardized form for RCTs was used to determine validity and eligibility for analysis.
Data extraction
Data extracted from each study included author, publication year, intervention and control group sizes, participant demographics (age, gender, illness type), treatment and follow-up duration, probiotic type, primary outcome definition, duration, and incidence. Data coding used MS Excel, cleaned and analyzed in RStudio using RMeta packages for summary statistics and plots.
Data synthesis and analysis
A random effects model was chosen for meta-analysis due to substantial study heterogeneity arising from diverse designs, demographics, controls, and interventions. The primary outcome summary statistic was risk ratio (RR) with 95% confidence interval, and I2 statistic assessed heterogeneity (significant if > 50%). Subgroup analyses examined sources and impacts of heterogeneity based on sample size, mean participant age, and number of strains administered.
Quality assessment
Bias assessment relied solely on information provided in published articles; additional data from design protocols or outcome measures were not sought, nor were individual participant data from authors. The Cochrane Risk of Bias tool (RoB-2) was used to assess bias across five domains: sequence generation, allocation concealment, blinding, completeness of outcome data, and selective outcome reporting. Ratings of low, some concerns, or high risk were assigned within each domain, and data were synthesized algorithmically in MS Excel before visualization using the RoBVis online tool. Publication bias was assessed using a funnel plot generated with RMeta software, and Egger’s linear regression analysis determined its strength (significant at p < 0.05).
Results
Results of study selection
Out of 2090 records found in the database search, 2017 titles were automatically removed based on study period and language criteria. After screening abstracts, 28 duplicates or studies indexed using different names or in various databases were excluded, leaving 15 accessible full-text articles. Among them, five were subscription-only, one was in Chinese without translation available despite the abstract being in English, and two were published in abstract series with inaccessible full content. The PRISMA flowchart summarizing the results of the search selection executed using the search strategy is shown in Figure 1.
Study range and characteristics
All included studies were RCTs involving adult patients, with a total sample size of 7427 (Table I). Participant numbers varied from 31 to 2941 per study, with a median participant age of 60.2 years, though one trial reported a median age of 34 years. Males were slightly overrepresented compared to females across most studies (53% vs. 47%), with two studies having more than 64% female participants. All studies recruited patients from hospital settings, with six conducted across multiple centers and nine as single-center studies. Three studies were conducted in specialized units such as ICU, renal, and gastroenterology departments. Commonly used antibiotics included β-lactams, cephalosporins, fluoroquinolones, and macrolides, primarily for respiratory and urinary tract infections, with a median antibiotic use duration of approximately 9.4 days.
Table I
Characteristics of randomized controlled trials of probiotics use for prevention and control of antibiotic-associated diarrhea and Clostridioides difficile infection
Participants were mostly split into probiotic treatment (3853 participants) or placebo/no intervention (3524 participants). Some studies explored dosage effects with three arms: two interventions and one placebo. Probiotics, mainly Lactobacillus, either alone or in combination with Bifidobacterium, Saccharomyces, or Enterococcus, were given within 24 to 48 h of starting antibiotics and continued for 3 to 14 days after stopping them. The duration of follow-up to identify the outcome of interest varied from 14 days to 72 days, with a median duration of 28 days. The outcome measure for AAD varied among studies. Definitions varied, with some using a stool frequency of 3 episodes per day as a cut-off, while others further characterized stool using the Bristol Stool Chart. Some studies incorporated clinical and laboratory tests for identifying Clostridioides difficile-associated diarrhea (CDAD). Assessment methods also differed, involving medical teams, admitting physicians, nursing staff, and even participants themselves. Only four studies reported CDAD alongside AAD. Secondary outcomes were diverse and had different definition terms.
Risk of bias within studies
The overall assessment of study quality was hindered by insufficient published information (Figures 2 A and B), resulting in a moderate rating for reporting quality. Ten studies had a low risk of bias, while five had a higher risk. Seven studies did not report how treatment allocation concealment was undertaken. Selective reporting of outcomes and attrition bias occurred in six studies. Most studies were double-blind, meaning there was a group that received a placebo, with one reported as triple-blind. Baseline characteristics of trial participants were largely similar across studies, with minor differences that were not statistically significant.
Effect of intervention on outcome
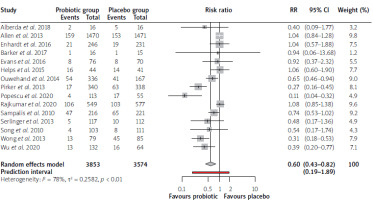
The results of the meta-analysis, including 15 studies, are summarized in a forest plot (Figure 3). Six trials showed a beneficial role of probiotic use in reducing AAD incidence; the rest (9) did not demonstrate a statistically significant advantage. Pooled summary estimates showed a reduction in the incidence of AAD in favor of probiotic use. The pooled RR, using the random effects model, was 0.6 (0.43–0.82). Prediction interval (0.19–1.89) offered insight into the variability of risk ratios across individual studies. There are studies where probiotic use could confer more than 80% protection against AAD, while others showed the risk of AAD being present despite the use of probiotics. It also means that future studies may fail to demonstrate any advantage of probiotics over placebo. Analysis of consistency of data across multiple studies demonstrated significant heterogeneity (I2 = 78%, p < 0.01).
Subgroup analysis
The significant heterogeneity presented in pooled results necessitated subgroup analysis, as portrayed in Figures 4 A–C. Studies were stratified into two based on sample size with a cut-off of 180 per trial group (Figure 4 A). This analysis demonstrated a lower risk ratio in studies with sample size below 180 (RR = 0.47, 95% CI: 0.29–0.75) as compared with those above 180 (RR = 0.75, 95% CI: 0.5–1.11). More heterogeneity was seen in the latter group (83% versus 53%). In Figure 4 B, the studies were grouped based on the mean age cut-off of 65 years. The pooled effect size of 11 studies that registered participants aged below 65 years was RR = 0.64 (95% CI: 0.48–0.85). The remaining four studies with participants aged 65 years and above had a pooled RR of 0.47 (95% CI: 0.17–1.30). Subgroup analysis based on a number of probiotic strains administered is illustrated in Figure 4 C. Ten studies that utilized one or two strains of probiotics had less effect on AAD than those (5 studies) that used more than two strains (0.6 vs. 0.9 vs. 0.4).
Analysis of secondary outcomes
Ten studies reported on the effect of probiotic use on the reduced incidence of CDI, ranging from 0.29% to 1.8% in the intervention group. In contrast, the control had incidences ranging from 0.87% to 13.3%. One study reported higher incidences both in the treatment group and control group (12.5% vs. 31.3%). Eight studies were retrieved to evaluate whether probiotics change the duration of diarrhea. The duration ranged from 1 to 8 days, most of which clustered around 4 days. Two studies that conducted a statistical comparison did not detect any significant difference between control and probiotic groups. Four studies inconsistently defined the severity of diarrhea. Key parameters considered were abdominal cramps or pain, bloating, and bloody stools. One study used bowel frequency and consistency, while two used duration of diarrhea as a marker of severity. Even with the latter criterion, one study used 3 days as a cut-off, while another used 5 days. Two studies demonstrated statistical significance favoring probiotics against placebo when bowel characteristics and bloating symptoms were used. A comparison of the two groups based on other abdominal symptoms lacked statistical significance. Only two studies assessed the dose effect of probiotics when compared to placebo. In both, three groups were utilized: high/double dose probiotic, low/regular dose probiotic, and placebo. They both demonstrated the advantage of higher doses over low-dose probiotics. Their comparison with placebo reached statistical significance in reducing the incidence of AAD, as demonstrated in Table II.
Table II
Summary of secondary outcomes as obtained from various trials
Assessment of publication bias
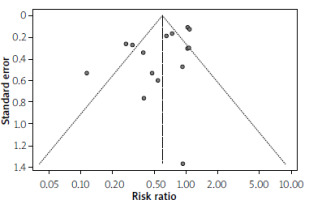
Inspection of the funnel plot (Figure 5) did not reveal any significant asymmetry, which implied the absence of publication bias or inflated influence from small studies. A linear regression analysis of funnel plot symmetry using Egger’s test was not significant (t = –2.38, d f = 13, p = 0.0331).
Discussion
The meta-analysis found that probiotic use reduces the incidence of AAD and CDI. Pooled results from 15 trials with 7427 participants suggest a beneficial effect of probiotic use in reducing the incidence of AAD among adult patients, with a summary effect size (RR) of 0.6 (95% CI: 0.43–0.82). Probiotics also reduced CDI incidence and severity of symptoms across the ten studies included in the review. Higher doses were more effective against AAD, but no statistical significance was found for diarrhea duration or other symptoms. The review encountered significant heterogeneity among the included studies, potentially affecting the strength of the findings. Variations in sample sizes, geographic locations, antibiotic usage, dosages, probiotic strains, diarrhea severity, and outcome definitions contributed to this heterogeneity. Subgroup analysis was attempted for the main outcome to explore these factors, while secondary outcomes required a systematic review due to potential bias in meta-analytic approaches.
The subgroup analysis based on sample size is hinged upon recommendations that studies aiming to reduce a relatively rare event such as AAD with an RR 0.58, should recruit more than 178 participants per group for adequate power (80%) [18]. This finding suggests a potential for overestimated effects in smaller studies, possibly due to the need to demonstrate significant effects. The second subgroup analysis focused on participants with a mean age of 65 years. Previous literature has stratified age groups into pediatric, adult, and elderly patients, with some studies showing limited effectiveness of probiotics in the elderly [19–21]. The same effect was observed by Jafarnejad et al.: probiotics reduced AAD incidence in participants aged 18–64, but no benefit was seen in those over 65 [22]. Although the risk ratio for studies with elderly participants was 0.47, the wide 95% confidence interval suggests reduced reliability due to heterogeneity in the study pool, indicating only marginal benefits of probiotics in the elderly. Finally, it has been suggested that the number of strain types administered could have a variable influence on AAD. Administering more than 3 strains of probiotics yielded favorable results, aligning with some of the existing research [23, 24]. Multistrain preparations enhance microbial diversity, beneficial for counteracting antibiotic effects in the gut lumen [25].
This research confirms prior studies suggesting that probiotics may help prevent AAD and CDI. In a recent meta-analysis including 42 studies and 11,305 participants, Goodman et al. found that co-administration of probiotics with antibiotics reduced the incidence of diarrhea by 35% (RR = 0.63, 95% CI: 0.54–0.73). Their subgroup analysis also established that higher doses and specific strains (Lactobacillus and Bifidobacterium) were advantageous [26]. Another meta-analysis of 63 RCTs indicated a reduced incidence of AAD with probiotic use (RR = 0.58, 95% CI: 0.5–0.68). Despite significant heterogeneity, subgroup analyses did not affect the results [18]. A Cochrane analysis revealed that probiotics reduced CDAD incidence (NNTB = 42 patients, 95% CI: 32–58). However, post hoc analysis showed benefits primarily in those with > 5% baseline CDAD risk [27]. Early probiotic intake (within 48 h of antibiotic initiation) is associated with lower AAD incidence and adverse events, supported by a separate meta-analysis [28, 29]. However, the efficacy of prolonged probiotic administration after antibiotic use remains unverified. Some reviews, like a Cochrane review involving 12,127 participants across 82 studies, found that probiotics offered little to no benefit in reducing the duration or occurrence of diarrhea lasting over 48 h. Additionally, they seemed not to reduce the duration of diarrhea [30]. While this study focused on treatment of acute infectious diarrhea, this could be extended to explain our findings in this review.
Limitations. While this study indicates a potential benefit of probiotics in reducing AAD, it has notable limitations requiring cautious interpretation. Expert opinions from study authors could have clarified methodological issues, but this was not pursued. The small number of included studies, attributed to strict eligibility criteria, limits generalizability. Additionally, determining probiotics’ impact on severity and duration of diarrhea, hospitalization length, and quality of life would enhance clinical utility, but many studies lacked such data, hindering comprehensive analysis.
Future prospects. This review underscores the potential of probiotics, suggesting that future trials should enroll sufficiently powered samples of at least 180 participants per arm to detect significant effects and clarify optimal dosage. Further research is also needed for identification of populations that benefit most from probiotics, determine superior probiotic strains, and explore their interactions with specific antibiotics.
In conclusion, our review of 15 studies found that probiotic use reduces the incidence of AAD and CDI. Pooled outcomes have demonstrated the beneficial effect of higher doses and multistrain combinations across the adult age span. Wide availability and their low cost lend credibility for their adjunctive use in ameliorating diarrheal episodes associated with antibiotic use.