Introduction
Biliary tract cancer (BTC) is a lethal malignancy originating in the bile ducts, the gallbladder, or the ampulla of Vater. It represents the second most common type of hepatobiliary cancer worldwide, with higher incidence rates in the Asia-Pacific region and in South America [1, 2]. Complete surgical resection remains the only curative treatment option for patients with BTC [3]. However, despite advanced surgical procedures and perioperative management, long-term outcomes of patients treated with surgery remain poor, with a 5-year overall survival (OS) rate of approximately 25–50% [4, 5]. Thus, significant efforts have been made to improve the early diagnosis and multimodal treatment, including chemotherapy [5, 6].
Factors predicting the survival of patients with BTC include tumour-node- metastasis (TNM) stage [7], neurovascular invasion, tumour differentiation grade, and resection margin status; however, each of these factors is typically revealed only during or after surgery. To guide patients’ stratification and risk-based treatment in BTC, several studies have investigated preoperatively available prognostic factors such as tumour markers, and inflammatory and coagulation parameters [8–11]. Nutritional status has also received increasing attention as an important factor predicting the survival of cancer patients [12, 13]; however, well-established, highly predictive nutritional parameters are necessary in clinical practice for patients with BTC.
The geriatric nutritional risk index (GNRI) is a simple nutritional assessment tool that is calculated from body weight, height, and serum albumin level. It was originally developed to predict the risk of morbidity and mortality in hospitalized elderly patients [14]. There is increasing interest in studying the prognostic relevance of GNRI in various malignancies. It has consequently been suggested that GNRI has prognostic value, in particular for patients with cancers of the digestive system such as oesophageal, gastric, colorectal, pancreatic, and liver cancers [15–22]. The prognostic value of GNRI in patients with BTC remains to be elucidated; however, a previous study reported that GNRI was an independent prognostic factor for postoperative survival in patients with gallbladder cancer [23].
Based on the hypothesis that the GNRI score is a prognostic factor for BTC, the present study investigated the association between preoperative GNRI and survival outcomes of patients with BTC undergoing surgical resection.
Material and methods
Patients, data collection, and ethics statement
Data from 250 consecutive patients with BTC who underwent surgical resection at our institute between January 2010 and June 2022 were retrospectively collected. The inclusion criteria were as follows: (1) histologically diagnosed as BTC; (2) underwent curative intent resection with no evidence of unresectability before surgery; and (3) with follow-up data. The exclusion criteria included the following: (1) surgery with palliative intent; and (2) missing important clinicopathological data due to incomplete medical records. A total of 213 patients were eligible and analysed. The primary tumour sites were as follows: intrahepatic bile duct (n = 43), hilar bile duct (n = 46), distal bile duct (n = 65), gallbladder (n = 28), and the ampulla of Vater (n = 31). One hundred and thirty-five (63%) patients who had biliary obstruction underwent biliary stenting as a bridge to surgery. Clinicopathological data including age, gender, American Society of Anesthesiologists physical status (ASA-PS) [24], body mass index, preoperative serum levels of carbohydrate antigen 19-9 (CA19-9) and albumin, and inflammatory markers, such as serum C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR), were collected. The neutrophil-to-lymphocyte ratio was defined as the ratio of absolute peripheral blood neutrophil and lymphocyte counts. The CA19-9 level was measured within one month before surgery (after biliary stenting), but other blood parameters were adopted on admission (one or two days before operation). Histopathological parameters included TNM stage according to the seventh edition of the Union for International Cancer Control TNM system [7], tumour differentiation, perineural invasion, and status of resection margin. Resection margins were classified as follows: R0, no residual tumour; R1, microscopic residual tumour; and R2, macroscopic residual tumour [7]. Ten patients who did not undergo lymphadenectomy (transduodenal ampullectomy for ampullary cancer, n = 7; cholecystectomy for gallbladder cancer, n = 3) were categorized as N0. Fifteen of 213 patients (7.0%) were found to have limited metastatic disease during surgical exploration (para-aortic lymph node metastasis, n = 6; liver n = 6; peritoneum n = 3). These patients underwent simultaneous resection of primary and metastatic lesions. The median follow-up time was estimated using the reverse Kaplan-Meier method [25]. Overall survival was calculated from the date of surgery to the date of last follow-up or death due to any cause. Relapse-free survival (RFS) was measured from the date of surgery to the date of relapse, including locoregional recurrence and distant metastasis. This study was approved by the Institutional Ethics Committee of Meiwa Hospital (approval no. 2022-39) as a retrospective analysis of the collected data in accordance with the ethical standards of the World Medical Association’s Declaration of Helsinki. Individual consent for this retrospective analysis was waived.
Calculation of geriatric nutritional risk index
We used the following GNRI formula: GNRI = 14.89 × serum albumin (g/dl) + 41.7 × present/ideal body weight (kg). The ideal weight was calculated from the Lorenz equation: for males: height – 100 – ([height – 150]/4), and for females: height – 100 – ([height – 150]/2.5). If the current body weight exceeded the ideal body weight, the present/ideal body weight value was set as 1 [14].
Statistical analysis
No statistical sample size calculations were conducted. According to a previous study, the cut-off value of GNRI was set at 98, and the patients were classified into a low (< 98) and high (≥ 98) GNRI group [14]. The cut-off values of blood parameters were determined according to the normal limit for the institute’s laboratory (CA19-9, 37 U/ml; albumin, 4.1 g/dl; CRP, 0.3 mg/dl). Median values were used to dichotomize other continuous variables, such as age and NLR, if there were no widely accepted cut-off values. Fisher’s exact test for categorical variables was applied to test for differences between groups. Overall survival and RFS following surgical resection were analysed by the Kaplan-Meier method, and survival curves were compared using the log-rank test. To identify risk factors associated with OS or RFS, Cox regression hazards models were performed, and univariate predictors with a p-value of less than 0.05 were included in the multivariate model. Multivariable-adjusted survival curves were also plotted. All statistical analyses were conducted using R 4.2.2 software (Foundation for Statistical Computing, Vienna, Austria), and a p value of less than 0.05 was considered significant.
Results
Geriatric nutritional risk index and clinicopathological characteristics
The study population consisted of 118 males and 95 females, with a median age of 72 years (range 39–88 years). Overall, 135 (63%) patients were classified into the low- GNRI group and 78 (37%) into the high-GNRI group. Table 1 summarizes clinicopathological data from each group. No significant differences in age, gender, ASA-PS, tumour location, NLR, T-stage, M-stage, tumour differentiation, and frequency of adjuvant chemotherapy were observed between the groups. However, the low-GNRI group had fewer obese individuals (p = 0.004), higher rates of patients with preoperative biliary stenting, hypoalbuminaemia (p < 0.001), elevated serum CA19-9 (p < 0.001), and CRP (p < 0.001) levels, lymph node metastases (p = 0.004), and perineural invasion (p = 0.020), and lower rates of patients with R0 resection (p = 0.042) than the high-GNRI group.
Table 1
Comparison of clinicopathological characteristics of the high- and low-geriatric nutritional risk index groups
Kaplan-Meier and Cox regression analysis for overall survival
The median follow-up time of all patients was 72.6 months, with a 95% CI: 53.7–84.4 months. Kaplan-Meier analysis and the log-rank test revealed that patients in the low-GNRI group had a significantly worse prognosis in terms of OS than those in the high-GNRI group (median = 31.4 [95% CI: 24.5–44.3] months vs. 83.5 months [95% CI: 50.7 months-not available]; p < 0.001) (Fig. 1 A). In univariate Cox regression analysis, CA19-9; CRP; pathological features, including T-, N-, and M-Stage; and GNRI were significantly associated with OS (Table 2). Multivariate analysis revealed that low GNRI was an independent prognostic factor for poor OS (hazard ratio [HR] = 1.731, 95% CI: 1.111–2.696; p = 0.015, Fig. 1 C), in addition to CA19-9 elevation (p = 0.008) and M1 Stage (p = 0.031) (Table 2).
Fig. 1
Kaplan-Meier survival curves showing overall (A) and relapse-free (B) survival for patients in the low- and high-geriatric nutritional risk index (GNRI) groups. Adjusted survival curves by Cox’s hazard regression model of overall (C) and relapse-free (D) survival for patients in the low- and high-GNRI groups
GNRI – geriatric nutritional risk index
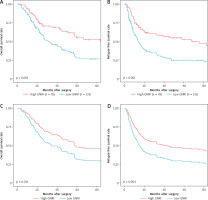
Table 2
Univariate and multivariate analyses of prognostic factors for overall survival
Kaplan-Meier and Cox regression analysis for relapse-free survival
The difference in RFS between the low- and high-GNRI groups was significant by Kaplan-Meier analysis (median = 13.6 [95% CI: 10.7–18.0] months vs. 67.5 months [95% CI: 20.8 months-not available]; p < 0.001) (Fig. 1 B). Multivariate Cox regression analysis revealed that a low PMTH was an independent prognostic factor for poor RFS (HR = 1.900, 95% CI: 1.231–2.931; p = 0.004, Fig. 1 D). Furthermore, intrahepatic cholangiocarcinoma (vs. distal cholangiocarcinoma); higher T-, N-, and M-Stage; and R1/R2 resection margin status were independent prognostic factors for poor RFS (Table 3).
Table 3
Univariate and multivariate analyses of prognostic factors for relapse-free survival
Discussion
In the present study, low GNRI was independently associated with poor OS and RFS in patients with BTC undergoing surgical resection, suggesting that GNRI provides valuable prognostic information preoperatively. A previous study of 202 patients with gallbladder cancer showed that patients with low GNRI had significantly poorer OS and RFS after radical surgery than those with high GNRI [23]; however, the present study is the first, to our knowledge, to examine the relationship between GNRI and survival outcome in BTC.
The exact mechanisms by which GNRI is related to survival among cancer patients remain unclear. Low GNRI depends on weight loss and hypoalbuminaemia; thus, it suggests malnutrition. In general, malnutrition is detrimental to the immune system, and impaired immune function affects cancer development and progression [18, 26]. In the present study, the low GNRI group had more advanced disease characterized by higher rates of CA19-9 elevation, lymph node metastases, and perineural invasion, as well as lower rates of R0 resection. This finding supports previous studies on oesophageal, gastric, and colorectal cancer patients, which reported that low GNRI was associated with a higher TNM stage and tumour markers, even though GNRI was an independent prognostic factor by multivariate analysis [15, 17, 19]. Furthermore, malnutrition is related to postoperative complications [18, 20, 21], increased chemotherapy toxicity [27], and shorter time to treatment failure [28], which may also lead to shorter survival.
Systemic inflammation is an important pathology contributing to malnutrition and worsened prognosis in cancer patients. Several inflammatory parameters have been reported to be useful as predictive indicators of long-term outcomes of patients with BTC such as NLR [29], platelet-to-lymphocyte ratio [30], and Glasgow prognostic score (consisting of a combination of CRP and albumin level) [9, 31]. However, these indicators are easily affected by some conditions, such as cholangitis, leading to biased results and difficulty in interpretation [29, 32]. Patients with BTC are prone to biliary bacterial infection preoperatively, in particular after biliary intervention for obstructive jaundice [33]. In the present study, patients in the low-GNRI group more frequently received biliary stenting and had elevated CRP levels than those in the high-GNRI group. Considering that the serum albumin level, a component of GNRI, can be reduced by inflammation, this study also analysed inflammatory markers (CRP and LNR) as possible confounding factors. As a result, multivariate analysis found that GNRI was associated with poor OS and RFS, regardless of the CRP or NLR value. Thus, GNRI may be a valid and robust index of nutritional status for predicting survival of patients with BTC.
The prevalence of malnutrition (as defined by a GNRI < 98) in our cohort was 63%, which was relatively high compared to that reported in previous studies in various kinds of cancers, which ranged from approximately 20 to 50% [15, 17, 19, 21–23]. This could be due to several factors, such as sample size, age group, tumour malignancy/aggressiveness, and the cut-off value used for GNRI. There was a lack of uniformity in the cut-off values of GNRI across the previous studies. In the present study, we adopted the most commonly used cut-off value, which was originally proposed by Bouillanne et al. in 2005 [14]. Further research to establish a generalizable, consensus-based cut-off for GNRI is necessary.
There were several limitations of this study. Firstly, it was a retrospective, single-institution study with a relatively small sample size, which had methodological limitations associated with selection bias and statistical measurements. Secondly, other nutritional parameters, such as prognostic nutritional index calculated by serum albumin level and absolute lymphocyte count, were not incorporated in the analysis. Assessing multiple parameters in combination may provide a more comprehensive picture of the nutritional status of BTC patients. Thirdly, only preoperative GNRI data were used for the analysis. Dynamic changes in GNRI during the follow-up period, which may be a better predictor of outcomes, were not examined in this study. Fourthly, the patients’ comorbidities and postoperative complications were not assessed and may have acted as confounding factors.
Conclusions
Our findings suggest the applicability of GNRI as a prognostic factor in BTC, and that preoperative low GNRI is associated with an increased risk of mortality and recurrence after surgical resection. As an easily accessible index in preoperative routine work, GNRI has potential value for determining which patients should receive perioperative aggressive nutritional support, more careful follow-up, and adjuvant treatment. Further studies with larger sample sizes are needed to validate our findings.








