Children frequently require sedation or anaes-thesia for magnetic resonance imaging (MRI) studies. This cumbersome, although non-invasive exami-nation is often performed in very young and sick patients and remains a challenge for anaesthesiologists. Elimination of child’s stress, body movement, and unnecessary interventions are important factors for its success [1].
General anaesthesia or deep sedation can improve the patient’s comfort and reduce movement, making the MRI examination easy and properly dia-gnostic. Intravenous techniques, especially with the use of propofol, have recently gained much popularity. This drug has been accepted for procedures outside the operating room, including a day case surgery. It decreases postoperative nausea and vomiting (PONV) and thus risk of dehydration, electrolyte abnormalities, and delayed discharge [2]. Pain on injection, apnoea and bradycardia are side effects of propofol [3, 4], and adjuvant agents, such as lidocaine, fentanyl, or ketamine are suggested to minimize these untoward reactions and reduce propofol requirements [5].
The new agent proposed for MRI sedation in children is dexmedetomidine – an a2 agonist – although bradycardia and hypotension after its use (especially with high doses) were noted [6]. In spontaneously breathing children sedated with dexmedetomidine infusion for MRI (1 or 3 µg kg–1 h–1), no untoward respiratory sequelae were observed [7].
To broaden the knowledge about the optimal method of sedation for MRI in children, we conducted a prospective, randomised study comparing two intravenous techniques – one based on propofol and the second on dexmedetomidine.
METHODS
After Institutional Review Board approval and informed consent from parents or guardians were obtained, hospitalized children aged 1–10 years with ASA status I or II scheduled for MRI examinations were included in the study. Patients with airway abnormalities, cardiac defects, or arrhythmias, and those who developed pathologic reactions after the studied medications, were excluded. They were randomly assigned (1 : 1) to receive either propofol (Group P) or dexmedetomidine (Group D), using the technique of sealed and consecutively numbered envelopes. Team members were not aware of the sedation choice until immediately prior to induction. Children fasted for 6 h (solids) and for 2 h for clear fluids. Premedication with midazolam (0.1 mg kg–1) and ketamine (1 mg kg–1) was given 2–3 min before the MRI procedure through an already present IV catheter. Atropine was not used. Sedation was induced with bolus of dexmedetomidine (Group D, 1 µg kg–1) followed by infusion of 2 µg kg-1 h-1, or with propofol (Group P, 1 mg kg–1) followed by an infusion of 4 mg kg–1 h–1. After induction children received 1 L min–1 oxygen flow through nasal cannulae. When children reached 4 points on the Ramsay Sedation Scale the MRI scanning was begun. No IV fluids apart from studied medications were infused. Oxygen saturation (SpO2), heart rate (HR), and non-invasive blood pressure (NIBP) were monitored and recorded every 5 min during MRI and after transfer to the post-anaesthesia care unit (PACU). End tidal CO2 monitoring was used in the first few cases, but the waveforms obtained were weak and values unreliable, so it was abandoned later. Any noted abnormalities and/or need for interventions was recorded. Awakening was confirmed when the child purposely opened their eyes and/or answered simple questions. Children with an Aldrete score of ≥ 9 were discharged to the unit of origin.
Statistical analysis
The Shapiro-Wilk test was used to test normality of data. The groups were compared by means of the c2 test with Yates correction for categorical data and the Mann-Whitney U test for continuous data. Results were expressed as medians and interquartile (IQR) – 25–75% quartile range for continuous variables or percentages for categorical variables. The significance of difference in number of interventions during anaesthesia was assessed using the two-tailed Fisher test. Patients’ HR, BP, SpO2 are presented as means of their measurements taken at 5 min intervals, divided by corresponding pre-procedural values. The results are therefore presented as decimals of baseline values. For all comparisons P-values of < 0.05 were considered statistically significant. Statistica V. 13.1 PL (StatSoft, Tulsa, Oklahoma, USA) was used.
RESULTS
We analysed 64 consecutive children sedated for an MRI scan in the period from 1st March to 30th April 2019. Their age ranged from 1 to 10 years (Table 1). Three patients were examined twice – the data from the first examination were used in the analysis. Two patients were excluded from the analysis – one because of the poor SpO2 signal from the cold extremities and the second due to incomplete data. Finally, 62 children were included in the analysis – 33 patients in Group D (M : 17; F : 16) with median age 4.03 years [IQR: 2.41–6.15], and 29 patients in Group P (F: 9; M: 20; median age 3.52 years) [IQR: 1.87–5.47]. The ASA status was similar in both groups and the demographic variables did not differ significantly (Table 1). Also, the baseline vital parameters showed no significant differences (P < 0.05).
TABLE 1
Demographic data
The scanned body parts are presented in Table 2. The median MRI study time was 38 min in the Group D (IQR: 33.0–42.0] and 43 min in the Group P (IQR: 35.0–53.0], P = 0.1.
TABLE 2
Scanned body parts
| Group D | Group P | |
|---|---|---|
| Central nervous system (CNS) | 14 | 12 |
| Facial bones | 2 | 3 |
| Chest | 2 | 1 |
| Abdomen | 7 | 5 |
| Other organs | 4 | 5 |
| Two body parts | 2 | 1 |
| Total body | 2 | 1 |
| MRI + CT | 0 | 1 |
During MRI 10 patients in Group P needed intervention by an anaesthesiologist (additional bolus medication – 5 cases, hypotension – 3, oxygen desaturation – 2), significantly more than in Group D, where only one intervention caused by desaturation was needed (P = 0.002) (Table 3). The slightly longer scan time in the Group P (NS) may be caused by the increased number of interventions during the imaging.
TABLE 3
Reasons for interventions
| Group D | Group P | |
|---|---|---|
| Desaturation | 1 | 2 |
| Hypotension | 0 | 3 |
| Need for bolus dose | 0 | 5 |
| Child’s body movement | 0 | 4 |
| Unplanned awakening | 0 | 1 |
The PACU awakening time was significantly longer in Group D (median 48 min [IQR: 28.0–65.0]) than in Group P (median 34 min [IQR:17.0–43.0]), P = 0.009. While undergoing the MRI scan both groups had similar values of HR, NIBP and SpO2 (Table 4). Four patients in Group D and 5 patients in Group P were admitted directly to their ward without entering the PACU, as they reached 9 points on the Aldrete’s scale after the scan.
TABLE 4
Median (IQR) values of SpO2, pulse rate, systolic (SBP) and diastolic (DBP) blood pressure during sedation for MRI. Results are presented as decimals of baseline values
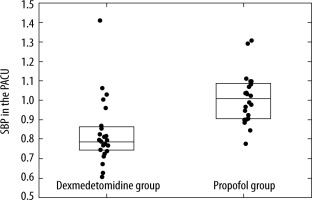
Patients from Group D had lower blood pressure values in the PACU compared with Group P (median SBP 0.78 [IQR: 0.75–0.86] of baseline and median DBP 0.73 [IQR: 0.62–0.93] in the Group D, in contrast to the Group P, where median SBP was 1.01 of baseline [IQR: 0.91–1.09] and median DBP was 1.00 [IQR: 0.90–1.06] (Figure 1).
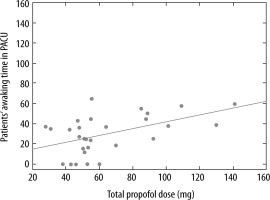
We did not find a correlation between the imaging time and the time of awakening, but there was a positive correlation between the total dose of propofol and the time of awakening in the PACU (rank Spearman correlation R = 0.44, P = 0.03) (Figure 2).
Discussion
Our study shows that both dexmedetomidine and propofol, after premedication with ketamine and midazolam, can be safely and reliably used for sedation in children with ASA status I or II undergoing MRI investigations. We chose smaller bolus doses at the beginning of procedures (1 µg kg–1 of dexmedetomidine and 1 mg kg–1 of propofol) because premedication with ketamine can spare the doses of other anaesthetics. Our dexmedetomidine dose was lower than in the study by Mason et al. [8], where the loading dose was 3 µg kg–1 followed by infusion with the rate 2 µg kg–1 h–1. The propofol bolus dose was also lower as compared with Johnson et al. [9], where a 2.2 mg kg–1 bolus was used, followed by infusion of 5.6 mg kg–1 h–1.
In the study by Mason et al. there was a 97.6% rate of successful sedation (able to complete the MRI protocol), although dexmedetomidine caused bradycardia in 16% of children. The use of anticholinergics to prevent or treat bradycardia related to dexmedetomidine administration is risky, as there are reports of profound transient hypertension when glycopyrrolate was used for this purpose [10]. In our patients no atropine or glycopyrrolate was used.
Many methods are suitable for achieving the goal of an unconscious and unmovable paediatric patient. Children undergoing MRI examinations often suffer from neurological diseases and are treated with various medications such as baclofen, diazepam, dantrolene, and/or anticonvulsant drugs, so the response to anaesthesia can be modified by an interaction with them. Inhalation anaesthesia with sevoflurane delivered by a face mask or laryngeal mask airway is still practised in some institutions. This is however related to problems with proper sealing of the mask and pollution of the MRI suite. Sevoflurane is known to be associated with emergence delirium and less likely with malignant hyperthermia [11, 12].
Pharmacokinetic characteristics of propofol, such as volume of distribution and clearance, are affected by body surface area (BSA), maturation of organ function, protein binding, and underlying disease. Children have a relatively large volume of distribution and clearance, and these parameters change during growth and maturation. Therefore, age and BSA should be included in formulas to estimate the propofol dose required for sedation in children [13]. Propofol was found useful in children for MRI anaesthesia, but respiratory depression and decreased blood pressure may reduce its applicability. We did observe more incidents of hypotension in group P during MRI.
Dexmedetomidine has been successfully used for sedation in children undergoing different procedures. It is the active dextro-isomer of the imida-zole compound medetomidine, and has selective a2-adrenergic activity with a differential specificity for the a2 : a1 receptors of 1620 : 1, compared with 220 : 1 for clonidine [14]. It has sedative, analgesic, and anxiolytic effects [15]. Its intraoperative administration reduces anaesthetic requirements and blunts the sympathetic nervous system response to surgical stimulation [16]. Its redistribution half-life is 9 min and elimination half-life is 110 min [15]. Dexmedetomidine can be used as a single agent for sedation, but then very high doses are usually required, resulting in prolonged discharge [6]. The main side effects are hypotension and bradycardia, which seem to depend on the infusion dose. With high dose boluses a paradoxical hypertension reaction was observed [17]. None of those happened in our patients. Combination with other drugs such as opioids, midazolam or ketamine is proposed. In our patients the effect of ketamine with midazolam given in premedication seems to have a positive clinical effect.
Sedation and anaesthesia for procedures such as MRI or CT in children are associated with risks of hypoxaemia and/or inadequate or failed sedation [18]. The first problem can cause dangerous sequelae for the brain, and the latter reduced the quality of images and increased personnel time with inconvenience for patients and families, when additional scans are needed. The main advantage of sedation with dexmedetomidine is the lack of respiratory depression and airway problems. Children with sleep apnoea undergoing MRI required fewer airway support interventions when dexmedetomidine at a 2 µg kg–1 IV loading dose followed by an infusion of 2 µg kg–1 h–1 was used for sedation, in comparison to propofol [19]. Similar results – more airway problems after propofol – were reported by Kang et al. [20]. In our study there was no significant difference between the respiratory events between dexmedetomidine and propofol groups, maybe due to the low dose of the drugs, especially propofol. On the other hand, there were more interventions in the propofol group, caused by body movement, additional boluses, hypotension episodes and premature awaking.
Comparison of dexmedetomidine and propofol for MRI sedation in children has already been performed in at least five studies followed by the meta-analysis by Fang et al. [21]. This analysis shows that the use of dexmedetomidine results in prolonged recovery in comparison to propofol. The largest difference in favour of propofol was observed by Wu et al. [22], who compared propofol and dexmedetomidine, after inhalation induction with sevoflurane. In that study the use of propofol resulted in a significantly shorter time for emergence from anaesthesia (21.2 vs. 39.9 min) and PACU stay (35.7 vs. 62.5 min). The meta-analysis by Tang also favours propofol in this respect [23]. Our study with a different regime also showed that dexmedetomidine can be responsible for longer PACU stay, but to a lesser extent, and the safe and predictable course of sedation convinced us that this drug is suitable for paediatric MRI.
It was proposed to give low dose dexmedetomidine (0.5 µg kg–1) as an adjuvant to propofol. Such addition should decrease the propofol requirement and reduce the need for airway support without prolonging recovery time [24]. Also, Boriosi [25] suggested that this combination results in fewer sedation-related complications, especially upper airway obstruction, although it is debatable which mechanism is in fact responsible for untoward respiratory effects [26]. Koruk et al. found that IV dexmedetomidine at a dose of 1 µg kg–1 combined with 1 mg kg–1 ketamine preserved haemodynamics, provided effective sedation, and facilitated more rapid recovery than midazolam plus ketamine in children anaesthetized for lithotripsy [27]. In the review by Mason and Lerman the authors suggest that dexmedetomidine with ketamine may be the key to maintaining respiratory drive as well as providing cardiovascular stability in infants and children [28]. We think that our patients benefited from ketamine and midazolam premedication, suggested by Warner et al. to be an optimal combination for that purpose [29].
Our study has several limitations. First, only children aged between 1 and 10 years were studied, so the results should not be applied to children younger than one year, and perhaps also older than 10 years. The number of studied patients was also small. Second, we excluded children with ASA status over II, upper respiratory infection or airway anomaly. The effects of studied drugs may be different for these children. Third, we did not use a sedation depth monitor to evaluate level of sedation, such as BIS [30]. Fourth, no CO2 measurement was utilized.
CONCLUSIONS
Our results show that after premedication with ketamine and midazolam, both dexmedetomidine and propofol are suitable for sedation of ASA I and II children examined by MRI, although dexmedetomidine causes longer recovery. Dexmedetomidine provided a smoother course of sedation with fewer interventions than propofol. Both agents had satisfactory sedative effects and were well tolerated. Further studies with a larger patient population and with different diseases may clarify the applicability of dexmedetomidine and propofol alone and in combinations for paediatric sedation outside the operating room.