Introduction
The rate of preterm birth ranges from 4 to 16% worldwide. In 2020, approximately 13.4 million neonates were born prior to completing 37 weeks of gestation [1]. Neonates born before 28 weeks of gestation are considered extremely premature. In these newborns, enlargement of the clitoris without a recognisable conventional cause is sporadically described.
Absence of a standard clitoromegaly definition, subjectivity in visual estimate, and inconsistencies in measurement technique make the objective assessment of clitoral enlargement in neonates and infants difficult [2]. Despite racial and ethnic differences [3, 4], clitoral length > 9 mm and width > 6 mm in full-term infants are considered abnormal. Recently, the first set of reference values for premature female neonates was published, defining clitoromegaly as clitoral length > 6.5 mm, with values ≥8 mm requiring rapid investigation [5]. Falsely perceived clitoromegaly in preterm newborns may be the result of complete clitoris development by the 27th week of gestation, with surrounding labial tissues and vulvar subcutaneous fat still underdeveloped at that time [2]. Hypoplasia of the labia majora, as a feature of popliteal pterygium syndrome (PPS), which is a rare autosomal dominant disorder with diverse clinical presentations including orofacial, skin, and genital abnormalities [6], may also contribute to the impression of an enlarged clitoris. While the presence of clitoromegaly at birth reflects excess prenatal androgen exposure of foetal (congenital adrenal hyperplasia), maternal, or placental origin, or rarely a non-hormonal condition (Beckwith-Wiedemann syndrome, clitoral cyst, or tumour), the exact underlying mechanisms for postnatal transient clitoromegaly in infants born prematurely are still insufficiently understood [7–10].
In this report, alongside presenting a case of an extremely premature twin infant with popliteal pterygium and transient clitoral enlargement associated with elevated circulating androgen levels, we highlight the current shortcomings in our understanding of sex hormone physiology in prematurity.
Case presentation
The patient is a female infant born to a 30-year-old mother, from a dichorionic diamniotic twin pregnancy. Because of gestational diabetes mellitus, the mother was treated with insulin. Tocolysis was initiated at 27 + 2 weeks of gestation, and subsequently accompanied by parenteral antibiotic therapy. However, preterm delivery occurred, and the patient was born vaginally at 27 + 5 weeks of gestation, as the first female twin (birth weight 992 g, birth length 34 cm, Apgar scores 7 and 9). Due to a prolapsed arm, the patient’s twin sister was delivered by caesarean section (birth weight 1095 g, birth length 36 cm, Apgar scores 7 and 9).
Immediately after birth, webbing of the skin from the ischium to the heel, on the back of the patient’s left leg was observed, with hypoplastic left labia majora, and without marked clitoromegaly. Cleft lip/palate or other visible anomalies of PPS were absent. Clinical exome sequencing performed subsequently did not reveal pathogenic variants in the IRF6 gene. The patient required non-invasive ventilation, surfactant and caffeine administration, ampicillin and gentamicin for suspected perinatal infection, insulin therapy for hyperglycaemia, as well as spironolactone and hydrochlorothiazide for bronchopulmonary dysplasia. Additional complications included neonatal jaundice, anaemia requiring red blood cell concentrate transfusion, patent foramen ovale, and patent ductus arteriosus (PDA). Fluconazole prophylaxis, oral erythromycin for feeding intolerance, and acetaminophen in the attempt of pharmacological PDA closure were also employed.
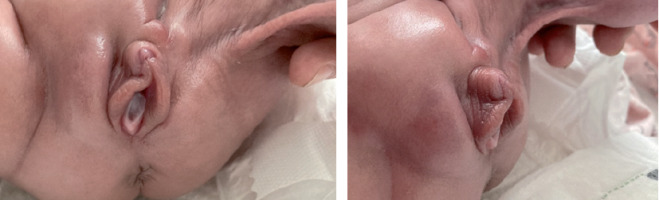
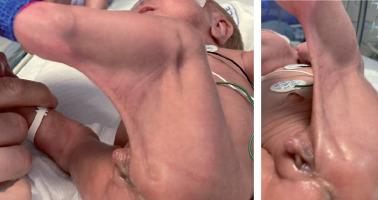
Unexpectedly, clitoris enlargement (length 1.2 cm, width 0.7 cm) was noted at the age of 48 days (corrected gestational age [CGA] 34 + 4 weeks) (Fig. 1). There were no other features of virilisation, nor obvious hypogastric/labial/upper leg swelling. 17-hydroxyprogesterone (17OHP) was unremarkable. However, luteinising hormone (LH), follicle stimulating hormone (FSH), testosterone, and dehydroepiandrosterone sulphate (DHEAS) were elevated (Table I). Anti-Müllerian hormone (AMH) was low, in accordance with XX female karyotype. Internal genitalia and left kidney were not visualised on ultrasound. High levels of LH, FSH, testosterone, and DHEAS subsequently normalised (Table I), which was accompanied by regression of clitoromegaly (Fig. 2). At the age of 4 months and 3 weeks (corrected age [CA] 2 months) the right ovary and ipsilateral segment of the uterus were ultrasonographically detected inside the patient’s inguinal hernial sac. A week later, repair of the right-sided inguinal hernia containing ovary and fallopian tube was performed. During surgery, however, the uterus was not visualised. Multi-step surgical correction of popliteal pterygium, transcatheter closure of patent ductus arteriosus, and repair of the left-sided inguinal hernia (CA 20 months 1 week) were performed as well.
Table I
Diagnostic workup at the onset of clitoromegaly and during follow-up
Figure 1
Clitoromegaly at the age of 48 days (corrected gestational age 34 + 4 weeks) in a premature infant with popliteal Pterygium
Interestingly, at the age of 1 month and 3 weeks (CGA 34 weeks), the patient’s twin sister was ultrasonographically diagnosed with simple ovarian cyst. She did not have clitoromegaly, hypogastric/labial/upper leg swelling, breast enlargement, or vaginal bleeding. Due to ovarian cyst enlargement and right-sided inguinal hernia, she was surgically treated at the age of 78 days (CA 38 + 6 weeks).
Discussion
Potential for clitoromegaly overdiagnosis in extremely premature female infants is high due to yet underdeveloped labial tissues and lack of vulvar subcutaneous fat [2]. Despite the popliteal pterygium with left labia majora hypoplasia, which could additionally enhance the impression of clitoral enlargement, in our patient the diagnostic criteria for clitoromegaly were fulfilled.
Enlarged clitoris present at birth, as an isolated feature or combined with various degrees of genital ambiguity, requires prompt evaluation. It is important to avoid congenital adrenal hyperplasia (CAH) related salt-wasting crisis, and to rule out the 46,XY difference in sex development (DSD) with significant undervirilisation. The diagnostic procedure usually involves detailed pre- and postnatal history, physical examination, electrolyte and hormone analysis, karyotyping, and diagnostic imaging. There are some reports of transient clitoromegaly in infants, in which the aforementioned causes have been ruled out, as was the case in our patient, but the underlying mechanisms remained obscure. Greaves et al. described 2 premature female infants with isolated clitoral enlargement and initial laboratory findings pointing either to less common forms of CAH or persistent foetal adrenal zone activity [7]. Due to the transient nature of clitoromegaly, its presence was attributed to the latter. Furthermore, in 3 premature female infants with clitoromegaly, who were initially treated as CAH, genetic analysis revealed no CYP21 mutations. Hydrocortisone therapy was discontinued, with unremarkable clinical course thereafter [8]. Dumont et al. presented a case of clitoral enlargement with increased androgen levels of unknown aetiology, which spontaneously resolved with normalisation of androgen levels [9]. Nerré et al. acknowledged ovarian androgen involvement in 2 extremely premature girls with transient clitoral hypertrophy [10]. Regardless of whether clitoromegaly was observed at birth [7, 10] or postnatally [8, 9], the unique characteristic of all the previously mentioned cases was the transient nature of clitoral enlargement.
However, the exact mechanisms behind this transient phenomenon in extremely preterm infants are not yet fully understood. It seems that premature birth interrupts the physiological course of foetal hypothalamic-pituitary-gonadal (HPG) axis maturation [11]. Gonadotropin levels normally peak at mid-gestation and subsequently decrease toward birth, probably as a result of gradual increase in the production of placental oestrogens toward the end of gestation. This trend is abruptly terminated by a preterm delivery, at a point of yet insufficiently matured HPG axis, which offers a reasonable explanation for exaggerated and prolonged postnatal gonadotropin surge in premature infants [12]. Only rarely, preterm ovarian hyperstimulation syndrome (POHS) characterised by high serum gonadotropin and oestradiol levels, ovarian cyst/cysts formation and hypogastric/upper leg swelling develops. In some premature female infants with accentuated LH surge or increased LH to FSH ratio, excessive androgen production was described [13]. The persistence of foetal adrenal zone may also create a hyperandrogenaemic state due to higher DHEAS levels [14, 15]. Our patient’s findings (transiently elevated gonadotropins, testosterone, and to a lesser degree DHEAS) are consistent with the aforementioned pathophysiological mechanisms. Other proposed mechanisms behind transient clitoromegaly include altered binding protein concentrations, differences in end organ sensitivity, and localised conversion of foetal steroids to more potent androgens in the genitalia [2]. It should be mentioned that our patient received red blood cell concentrate transfusion in NICU. Although a case of a female neonate who developed clitoromegaly during the second month of life after repeated whole blood transfusions from her father was described [16], the authors of a recent retrospective multicentre cohort study concluded that sex hormones delivered via plasma transfusions from adult male donors do not significantly impact the circulating plasma hormone concentrations in preterm infants [17].
We were not able to visualise our patient’s ovaries at the time of clitoromegaly onset, but approximately at the same time, a large simple cyst of the right ovary was detected on abdominal ultrasound in the patient’s twin sister. She developed neither POHS nor clitoromegaly, but due to the cyst enlargement she was eventually surgically treated. As mentioned before, our patient and her sister were fraternal twins, and they had no major differences in their clinical courses regarding prematurity and NICU stay, allowing us to speculate that their phenotypic variability was genetically determined. Because recent discoveries have pointed to androgen-led ovarian programming and its possible association with subsequent development of conditions such as polycystic ovary syndrome (PCOS) [18], dedicated follow-up of female neonates with intrauterine/early postnatal androgen exposure is required.
In conclusion, development of the HPG axis in the prenatal period and early infancy is influenced by premature birth, which interrupts this physiological process in its inception. Consequently, some extremely premature infants present with clitoromegaly, which can be transient. After exclusion of CAH and DSD, observation is the most appropriate strategy, because hyperandrogenism and clitoromegaly usually regress with time.

 ENGLISH
ENGLISH