Introduction
Flow cytometry (FC) is a single-cell analysis technique routinely used in diagnostic procedures of hematological and immunological disorders. The clinical use began in the 1980s, from diagnosis of patients with acquired immunodeficiency syndrome (AIDS) [1]. The technique is still an inseparable tool for monitoring CD4+ T cell count in patients with AIDS, as a predictor of disease progression [2]. Currently, the applications of FC are significant, and it constitutes a fundamental tool assisting the diagnosis and monitoring of secondary (SID) and primary immunodeficiencies (PID) [3, 4] as well as in hematological disorders, e.g. leukemias [5, 6]. Less commonly, FC is a helpful tool in the diagnosis of infectious diseases [7], such as Lyme disease [8], allergology (e.g. basophil activation test) [9, 10], and reproductive health.
Primary immunodeficiencies are a heterogenous group of genetically determined disorders which cause an impaired, improper, or absent immune response from one or more leucocyte compartments. Primary immunodeficiencies commonly manifest recurrent infections [16]. Over 400 gene mutations have been associated with the occurrence of PID [17]. Mostly, PIDs are associated with diagnosis in infancy or childhood. Due to a better understanding of these diseases and a wider range of tests available for diagnosis, the number of inborn immune disorders is rising every year [18]. However, in the last decades more attention has been given to cases of PID in adults [19]. Data indicate that 40-60% of patients with PID are diagnosed as an adult [20, 21], with 8% of the patients being older than 65 years [21]. PIDs that have an onset in adulthood are different from those diagnosed in childhood, e.g. thymoma with immunodeficiency (infancy Good’s syndrome). Most widespread are immunoglobulin (Ig) A deficiency, common variable immunodeficiency (CVID), late onset combined immunodeficiency (LOCID), idiopathic CD4+ lymphocytopenia, and GATA2 deficiency [21].
Common variable immunodeficiency, alongside selective IgA deficiency, is the most common immune deficiency, with heterogenous etiology, respecting adults. Recent retrospective studies have shown that the median age in adult male patients is around 30 years, 33.5 years for females, and the average age of diagnosis CVID is around 35 years [20–22]. The onset of symptoms preceded diagnosis of CVID by 6-10 years [22–25]. In addition to clinical symptoms of CVID, such as recurrent infections, enlarged lymph nodes, persistent inflammation, patients have a deficiency or an absence of serum IgG (below 2 standard deviations of the mean according to age) and IgA or, in minority, IgM levels, as well as a poor response to vaccines [26–28]. Importantly, in the diagnosis of CVID, in addition to clinical manifestations and determination of antibody levels, flow cytometric phenotyping of lymphocyte subsets and evaluation of peripheral maturation of B lymphocytes are recommended [22]. Reduced CD4+/CD8+ ratio, lower numbers of CD45RA+ T helper lymphocytes, lack or low numbers of B cells, elevated CD19+CD21low B lymphocyte subset, lack or low numbers of class-switched memory B lymphocytes (CD19+CD27+IgM−IgD−), and elevated transitional B cells (CD19+CD38++IgM−) were observed in CVID patients [23, 27-32].
Evaluation of lymphocyte subsets is one of the most used laboratory techniques in the diagnosis of immunodeficiencies, and helps to distinguish between primary and secondary immunodeficiency [30, 33]. The method is rapid and helps to choose further steps in the diagnostic process, e.g., targeted genetic testing; thus in our research we used flow cytometry to establish reference values according to age for men and women. Primarily, we focused on establishing reference values of relative frequencies and absolute counts of T lymphocytes (including CD8 and CD4 subsets), B lymphocytes (including transitional, naïve, marginal zone-like, class-switched memory, CD21low B lymphocytes, and plasma blasts), NK cells, and NKT lymphocytes, in healthy adults. Considering the wide onset age range, and evidence that women are diagnosed later than men [36], we aimed to assess differences in lymphocyte populations regarding sex and age.
Material and methods
Ethics approval
The Bioethics Committee of the Medical University of Warsaw approved the study. All measurements, interventions, and blood collections were performed after obtaining informed consent. The number of the Bioethics Committee statement is AKBE/83/2023.
Subjects and sample
Peripheral blood samples stored in 1.3 ml EDTA anticoagulant tubes were commercially obtained from the Regional Blood Centre in Warsaw. All participants had to meet the health criteria for healthy blood donors and were more than 18 years old at the time of sample collection. Finally, a group of 61 volunteers (31 women, 30 men) was included in the study. Information about age and gender was collected. All tests were performed within 4 hours of specimen collection.
Immunophenotyping
Multitest
Peripheral EDTA blood samples were mixed gently. 50 µl aliquots were transferred into Trucount tubes (BD Biosciences, USA) via reverse pipetting and 20 µl of anti-body cocktail – BD Multitest 6-color TBNK (BD Biosciences, USA), containing CD3−FITC/CD16+CD56−PE/CD45−PerCP−Cy5.5/CD4−APC/CD19−PE−Cy7/CD8−APC−Cy7, was added to each tube, then vortexed gently for 3-5 seconds and incubated at room temperature in absence of light for 15 minutes. After incubation, 450 µl of BD FACS Lysing Solution (BD Biosciences, USA) was added and left for further incubation (15 min, no light, room temperature) to lyse erythrocytes. A Becton Dickinson FACSCanto II cytometer (BD FACS Canto II, Becton Dickinson, Franklin Lakes, NJ, USA) was used to collect data, and then analysis was performed with BD FACS Diva 6.1.3 software. For an example of analysis of the results, see Supplementary Materials Figure S1.
Staining for B lymphocyte subsets
Prior to the experiment, each antibody was titrated to determine the concentration with the highest signal-to-noise ratio (Table 1). Compensation was calculated using single antibody-stained live peripheral blood cells in previously determined concentrations. Additionally, fluorescence-minus-one (FMO) tests were performed to determine cut-off values of antigen-positive cell populations. Sample preparation and staining were performed according to the procedure described by Piątosa et al. [34]. In order to minimize the staining interference of cell-bound antibodies, 300 µl aliquots of blood were washed in PBS containing 2% bovine serum and 0.1% sodium azide, and after centrifugation the supernatant was discarded. This step was repeated twice. Erythrocyte and leukocyte precipitate was resuspended in 200 µl of PBS containing 0.1% sodium azide. Aliquots of 100 µl were transferred to tube 1 and tube 2 and monoclonal antibodies were added as described in Table 1. Both tubes were incubated for 30 minutes at 4 degrees Celsius in the dark, then 1 ml of BD FACS Lysing Solution was added to each tube and incubated for 15 minutes, at room temperature, in the dark. Then, cells were washed twice and resuspended in 180 µl of PBS supplemented with 0.1% sodium azide.
Table 1
Volumes of antibodies that were added to each tube, established by previous titration experiments; BD – Becton Dickinson – manufacturer
Data acquisition
Cell readouts were acquired using a Becton Dickinson FACS Canto II cytometer (BD FACS Canto II, Becton Dickinson, USA). For automatic calculation of lymphocyte absolute count in a Multitest Trucount tube, FACS Canto Software (Becton Dickinson, USA) was used. Further analysis was performed with BD FACS Diva 6.1.3. software (Becton Dickinson, USA). Examples of data analyses are shown in supplementary data, Figure S1 for Multitest and Figure S2 for B cell subclasses, respectively.
Absolute counts of lymphocyte subpopulations were calculated as:
lymphocyte absolute count cell ml 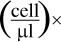 percentage of lymphocytes of lymphocyte subpopulation (%),
percentage of lymphocytes of lymphocyte subpopulation (%),
and absolute counts of B lymphocyte subpopulations were calculated as:
B lymphocyte absolute count cell ml 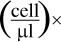 percentage of lymphocytes of B lymphocyte subpopulation (%).
percentage of lymphocytes of B lymphocyte subpopulation (%).
Statistical analysis
For each data set of lymphocyte subpopulation, the mean, standard deviation, standard error, median, and 5-95 percentile range were calculated for the 1) whole studied population and 2) with distinction for males and females. Data are shown in the tables. To analyze the differences between the male and female groups, an unpaired Student’s t-test was used since all data sets were bigger than or equal to 30. To assess the correlation of each studied subpopulation with age, the Spearman correlation test was performed. The α level was set at 0.05; p-values below 0.05 were considered statistically significant. All statistical analyses were conducted with GraphPad Prism 8.3.4 and the power of each Student’s t-test was determined with PQStat 1.8.0.
Results
A summary of our results is presented in Table 2 and Table 3 as mean, standard deviation, standard error, median, and reference range presented as 5 to 95 percentiles.
Table 2
Summary of distinct leukocyte population frequencies and absolute counts from Multitest
Table 3
Summary of distinct B lymphocyte population frequencies and absolute counts
In the studied population, we found a higher frequency of lymphocytes in the female volunteers’ group than in the male one (test power = 0.5082), higher frequency (test power = 0.7501) with higher absolute count (test power = 0.5488) of T helper lymphocytes, and higher CD4/CD8 ratio (test power = 0.5319) in the female group. In the male population, we found a higher frequency of CD4−CD8− T cells in comparison to the female group (test power = 0.4473).
Considering B lymphocyte subpopulations, we found the following differences between male and female populations: lower frequency of naïve B lymphocytes (test power = 0.9656) and transitional B lymphocytes (test power = 0.8091) among the female group in comparison to men; higher frequency of marginal zone-like B lymphocytes (test power = 0.7221), class-switched B lymphocytes (test power = 0.8672), CD21low B lymphocytes (test power = 0.6122) in the female in comparison to the male group. Interestingly, no difference was found in the absolute counts of the mentioned subpopulations between these two groups, except for transitional B cells, which were higher in the male group (power = 0.6132).
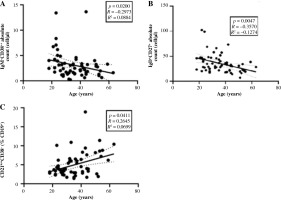
We found that the populations of CD4+CD8+ T and NKT lymphocytes had a weak positive correlation with age, and the population of CD4−CD8− T cells had a weak negative correlation with age for both frequency and absolute count (Fig. 1A, C). A CD4−CD8− T lymphocyte correlation with age was observed in the female population, whereas the same analysis showed no significance in the male group (p = 0.14, R = –0.2719). Additionally, among the studied B lymphocyte subsets, we found a negative correlation of absolute counts of marginal zone-like B cells (CD19+CD27+IgD+) and plasmablasts (CD19+-CD38++IgM−) with age (Fig. 2A, B) and a positive correlation of CD21lowCD38− B cell frequency with age (Fig. 2C). Surprisingly, in the male group we found no correlation of any studied B lymphocyte subset with age and shifts in the subsets were observed in the female group only.
Fig. 1
Correlation of distinct lymphocyte subpopulations. A) Correlation of CD3+CD4+CD8+ T lymphocyte frequency; B) Correlation of CD3+CD4+CD8+ T absolute count with age; C) Correlation of CD4−CD8− T lymphocyte frequency with age; D) Correlation of CD4−CD8− absolute count with age; E) Correlation of CD3+CD56+ cell frequency with age; F) Correlation of CD3+CD56+ cell absolute count with age. Spearman correlation tests were performed due to the ordinal character of the age. The studied population consists of both men and women, N = 61
NK-T – natural killer T lymphocytes
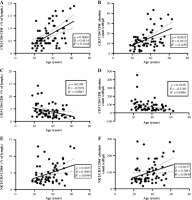
Fig. 2
Charts represent the correlation of distinct B lymphocyte subpopulations with age. A) Correlation of plasmablasts absolute count with age; B) Marginal zonelike B cell absolute count with age; C) CD21lowCD38− B lymphocyte frequency with age. Spearman correlation tests were performed due to the ordinal character of the age in this experiment. The population showed on the graphs consists of both men and women, N = 61
Discussion
Over the last thirty years a few similar studies have been performed in various parts of the globe, differing in the equipment and repertoire of antibodies used. One of the recent congruous studies was performed in Germany on healthy hospital staff volunteers, where a wide range of lymphocyte counts and frequencies were studied regarding immunosenescence, and differences in reference range according to gender [35]. The reference ranges of T lymphocytes in the mentioned study were similar to our results, excluding the CD8+ T lymphocyte percentage, which in our study was lower, probably because we distinguished and measured the population of CD4+CD8+ cells, which was not included in the study of Kverneland et al. [35]. Additionally, we achieved narrower reference ranges, while the cohort size was comparable, which suggests that our studied population was more homogeneous. Kverneland et al. did not find any of the gender-related differences in the proportions of B lymphocyte subsets that we found, with the exception of the percentage of transitional B lymphocytes, which we found to be higher in males, whereas Kverneland et al. found the reverse dependency [35].
Similar studies performed in France showed that women had significantly lower levels of CD4−CD8− and CD8+CD4+ T lymphocytes, naïve and transitional B lymphocytes, and higher levels of T lymphocytes, B lymphocytes, including class-switched B lymphocytes, and NK cells in comparison to the male group [36]. Some of the findings confirm our observations, e.g. elevated lymphocyte levels, class-switched B cells, and diminished transitional and naï ve B cell levels. We did not confirm some of the differences. The results we obtained seem to be coherent with other published papers regarding means and reference ranges of percentages and absolute counts of studied immune cells [35, 37-40]. Concerning B lymphocyte subsets, our results were consistent with the Piątosa et al. study, performed on a group of young Polish adults and adolescents (more than 16 years old), although our reference ranges were wider and the age of participants was higher [34]. Shahal-Zimra et al. observed a lower average proportion of lymphocytes (28.43 ±6.04% vs. 37.80 ±8.19% of WBC) and a lower number (193.20 ±102.00 cells/µl vs. 278.12 ±118.84 cells/µl) as well as proportion (9.82 ±5.05% vs. 14.08 ±5.42%) of NK cells in comparison to our observations [37]. Likewise, Valiathian et al. reported lower levels of NK cells [39]. In the research performed by Al-Thani et al., the absolute count of CD8+ T lymphocytes was higher (967 ±364 cells/µl vs. 499.97 ±171.66 cells/µl) than in our research [38].
Our study confirmed previous observations, where females tended to have higher proportions of CD4+ T lymphocytes in comparison to males [35-38, 40-43]. Nonetheless, the observation is not reproduced in each study [39]. Consequently, a replicated elevated CD4/CD8 ratio in the female population was also found [35, 36, 39, 41, 43]. However, we failed to verify the finding that CD8+ T lymphocyte frequency is higher in the male than in the female population, which was reported in several research papers [35, 37, 41]. Nevertheless, a few other studies did not find that dependency [42–44]. The discrepancies in these results might depend on the origin of the studied population.
Our results showed age-dependency of NKT-like cells, CD4−CD8− and CD4+CD8+ T lymphocytes and IgD+-CD27+CD21low B lymphocytes as well as plasmablast frequency and absolute count. In the work of Apoil et al., negative correlations of CD4−CD8− and plasmablasts with age were observed, similarly to our work [36]. Double-negative T lymphocytes are often overlooked in publications establishing reference ranges. In a recent study by Villegas-Valverde et al., it was noted that the population might have a negative correlation with age, significantly deteriorating after 50 years old, which supports our findings [45]. However, in our research, it concerns only the female population, while Villegas-Valverde et al. suggested that the correlation occurs in both groups, males and females [45]. It was reported that plasma and memory B cells decrease with age [46]. We partially confirmed that phenomenon in our study, since we found a negative correlation between age and memory CD27+IgD+ B lymphocytes. We noted the expansion of double-positive CD4+CD8+ T lymphocytes with age, as was reported by Laux et al. [47]. However, the double positive CD4+CD8+ T lymphocyte population is still poorly studied. The data of the role of CD4+CD8+ are controversial; cytotoxic and suppressive activity was reported [48]. Nevertheless, the population has been associated with numerous types of cancer and infectious diseases [48].
In previous studies concerning immunosenescence it was found that CD27+ B cells and exhausted memory B cells decline with age, which was reflected in our study, and CD21lowCD38− B lymphocyte count is higher with age [49]. The data we gathered indicating a different composition of B lymphocyte subsets regarding sex seem to support the general observation that women tend to have a stronger immune response to infection and a better response to vaccination than men [50, 51]. The observation is usually explained by the immunosuppressive influence of testosterone, while inter alia estradiol enhances the survival of naïve B cells and contributes to the production of high-affinity immunoglobulins, which might be supported by our data showing higher proportions of class-switching B cells in the female group [51, 52]. Moreover, the age-related changes in the composition of B lymphocytes that we observed in females, but not in the male population, might be explained by hormonal changes in the post-menopausal phase in females [52]. Additionally, elevated proportions of naïve and transitional B lymphocytes have been associated with a higher survival rate of allogenic transplant, due to elevated immunoregulatory subsets [53]. This might also reflect poorer humoral immune responses in men.
We failed to find other studies that would reflect our findings in this matter, since only a small number of researchers have carried out such analyses.
Conclusions
There is a great benefit of better reference adaptation of reference ranges regarding age and sex, since peripheral blood lymphocyte populations are not distributed equally among men and women, which should be taken into consideration during diagnosis of PID or SID. Our results may contribute to a better understanding of immunosenescence and sex-related susceptibility to various diseases as well as secondary immunodeficiencies.


