Introduction
Systemic sclerosis (SSc), also known as scleroderma, is a complex immune-mediated rheumatic disease characterized by the fibrosis of the skin and internal organs and vasculopathy [1]. Nowadays, epithelial cells have been proven to be one of the critical drivers and/or modifiers in SSc development [2]. Scleroderma keratinocytes could promote the activation of fibroblasts in a transforming growth factor-β (TGF-β)-independent manner [3]. It has been reported that there is a delay in the expression of terminal differentiation markers, keratins 1 and 10, in the scleroderma keratinocytes [4]. Similarly, involucrin is also one component of the cornified envelope, but it is an early terminal differentiation marker [5]. In the epidermis of SSc patients, involucrin forms thicker bands in comparison with healthy controls, which suggests a delay in the final stages of the terminal differentiation process [6].
Interleukins (ILs), tumor necrosis factor-α (TNF-α), transforming growth factor (TGF-β1), endothelin-1 (ET-1), interferon-γ (IFN-γ), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) – BB and cytokines are overexpressed in the scleroderma patients [7]. In addition, the Rho signalling pathway is pivotal in the pathogenesis of fibrosis, inflammation, and vascular abnormalities [8].
Aim
The current study was conducted to compare the differentiation of the skin of scleroderma patients and healthy controls by analysis of the involucrin levels. Additionally, we investigated the role of Y-27632, a potent ATP-competitive inhibitor of Rho, in the involucrin expression in both normal and scleroderma keratinocytes.
Material and methods
Human tissues
Our study was approved by the Human Research Ethics Board of the Second Affiliated Hospital, Zhejiang University School of Medicine (reference number: 2010-109) and was conducted according to the principles of the Declaration of Helsinki. A total of 12 skin samples from the forearm of SSc patients without limited forms (8 females with a median age of 35 years [range: 20 to 62]) and 12 age-matched healthy controls (8 females with a median age of 39 years [range: 24 to 61]) were included. All subjects had signed written informed consent. None of the patients had received any therapy for scleroderma 1 month before the biopsy. The biopsies were taken within the scleroderma lesions. One part of the specimens was quickly frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, CA, USA) and stored at –20°C. The other part was put into 0.5% dispase (Gibco, Invitrogen, USA) immediately to isolate the epidermis from the dermis.
Indirect immunofluorescence assay
Immunofluorescence (IF) assay was performed as previously described with some modifications [9]. Briefly, tissues were cut into 4-µm sections, placed on slides, then fixed in 4% paraformaldehyde buffer for 20 min at room temperature and put into 95°C sodium citrate buffer (10 mM, pH 8.5) for another 20 min. Next, the sections were incubated overnight with primary rabbit anti-involucrin antibody (Santa Cruz Biotechnology, USA) and cy3-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 2 h under light protection at room temperature. The nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI, Roche Diagnostics, Mannheim, Germany) for 20 min at room temperature. Each case had a negative control incubated with non-immune rabbit IgG (Sigma-Aldrich, USA).
Keratinocyte culture
The isolation and culture of primary keratinocytes were carried out as previously described [9]. The second or third generation of normal and scleroderma keratinocytes were used in this study. All the experiments were replicated three times. When grew to 80–90% confluent, the keratinocytes were incubated in low-Ca basal defined K-SFM overnight. Then, one part of the cells was incubated with IL-1β, IL-4, IL-6, IL-10, IL-17A, TNF-α, ET-1, TGF-β1, IFN-γ, VEGF165, and PDGF-BB (PeproTech , Rocky Hill, NJ, USA) at 25 ng/ml for 24 h. The other part of the cells was pre-treated with Y-27632 (Cayman Chemical, Ann Arbor, MI, USA) at 10 ng/ml for 2 h, and incubated with IL-13, ET-1, and IFN-γ (PeproTech, Rocky Hill, NJ, USA) for another 24 h.
Western blot
Total protein was extracted as previously described [10]. The protein was separated by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to the nitrocellulose filter membranes (Whatman, USA). Membranes were blocked for 1 h at room temperature and incubated with primary rabbit involucrin antibody (1 : 200) overnight at 4°C and HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 2 h at room temperature. After washing, immunoblot was developed with enhanced-chemiluminescence (ECL) reagents (Millipore Corporation, MA, USA) according to the manufacturer’s protocol. A mouse monoclonal anti-β-actin (SC-47778, Santa Cruz Biotechnology) was used as a loading control. For the densitometry analysis of involucrin/β-actin, Gel-Pro Analyzer software V6.0 (Media Cybernetics, MD, USA) was used.
Results
Immunolocalization and decreased involucrin expression in normal and scleroderma epidermal keratinocytes
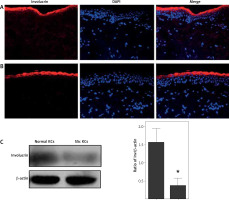
Immunofluorescence showed that involucrin was localized at the granular layer and upper stratum spinosum of normal and scleroderma epidermis, not in basal and suprabasal prickle keratinocytes (Figures 1 A, B). At the protein level, the involucrin expression was significantly decreased in scleroderma keratinocytes compared to normal keratinocytes (p < 0.05, Figure 1 C).
Figure 1
Immunolocalization and expression of involucrin in healthy controls and scleroderma patients. Immunolocalization of involucrin in (A) healthy controls and (B) scleroderma patients. Involucrin is indicated by red fluorescence. Nuclei are stained with DAPI (blue signal). C – A representative immunoblot results of involucrin protein in the cultured normal keratinocytes and scleroderma keratinocytes. The columnar section is the relative quantitation of involucrin protein levels in 8 different samples, which are shown after normalization to the endogenous control of β-actin. *p < 0.05
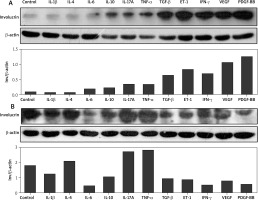
Regulation of IL-1β, IL-4, IL-6, IL-10, IL-17A, TNF-α, TGF-β1, ET-1, IFN-γ, VEGF165, and PDGF-BB and the production of involucrin in normal and scleroderma keratinocytes
In normal keratinocytes, IL-6, IL-10, IL-17A, TNF-α, especially TGF-β1, ET-1, IFN-γ, VEGF165, and PDGF-BB increased the production of involucrin at the protein level in comparison with the control group. However, compared with the control group, there were no significant changes in the production of involucrin induced by IL-1β and IL-4 in normal keratinocytes (Figure 2 A). Interestingly, in scleroderma keratinocytes, their effects were different. IL-1β, IL-6, IL-10, TGF-β1, ET-1, IFN-γ, VEGF165 and PDGF-BB decreased the involucrin levels, while IL-4, IL-17A and TNF-α promoted the involucrin expression (Figure 2 B).
Figure 2
Effects of IL-1β, IL-4, IL-6, IL-10, IL-17A, TNF-α, TGF-β, ET-1, IFN-γ, VEGF, and PDGF-BB on the expression of involucrin in the cultured normal keratinocytes and scleroderma keratinocytes. Both normal keratinocytes (A) and scleroderma keratinocytes (B) are starved overnight and incubated with these cytokines for 24 h
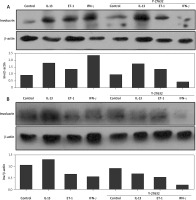
The role of Rho-inhibitor (Y-27632) in the production of involucrin in normal and scleroderma keratinocytes
IL-13, ET-1, and IFN-γ at 25 ng/ml up-regulated involucrin at the protein level in normal keratinocytes after intervention for 24 h. Inhibition of Rho signalling showed no effect on the production of involucrin in both normal and scleroderma keratinocytes. Rho inhibitor did not change IL-13- and ET-1-induced increase of involucrin in normal keratinocytes and ET-1- and IFN-γ-decreased involucrin in scleroderma keratinocytes. However, contrary to the upregulation, IFN-γ and IL-13 remarkably downregulated the expression of involucrin in normal (Figure 3 A) and scleroderma keratinocytes (Figure 3 B) after Y-27632 intervention, respectively.
Figure 3
Effects of IL-13, ET-1, IFN-γ, and Rho-inhibitor (Y-27632) on the expression of involucrin in the cultured normal keratinocytes and scleroderma keratinocytes. Both normal keratinocytes (A) and scleroderma keratinocytes (B) are starved overnight, pre-treated with Y-27632 for 2 h, and incubated with these cytokines for 24 h
Discussion
Epithelial-fibroblast interaction now appears to be relevant to the pathogenesis of scleroderma [4]. Epithelial cells promote fibroblast activation in scleroderma [3]. Involucrin is an early terminal differentiation marker of keratinocytes [5]. In normal human epidermis, involucrin initiates its action in the early spinous layer and is maintained in the granular layer [11], which is confirmed in our results, in both normal skin and scleroderma skin. However, in scleroderma epidermal keratinocytes, the involucrin expression is significantly decreased, which suggests that the terminal differentiation may be delayed in the scleroderma epidermis.
Pro-inflammation cytokines, such as IL-1, IL-4, IL-6, IL-10, IL-13, IL-17A, ET-1, TNF-α, TGF-β1, IFN-γ, VEGF, PDGF-BB, are shown to be elevated in the serum or lesions of the scleroderma patients [7]. Therefore, we examined the role of these cytokines in keratinocyte differentiation. The involucrin expression level was significantly decreased by IL-6, IL-10, TGF-β1, IFN-γ, ET-1, VEGF, and PDGF-BB, suggesting that the downregulation of involucrin in scleroderma skin may be due to the overexpression of these cytokines. It seems that even in the same cell type, for example, keratinocytes, if in different phenotypes, they may respond differently or completely reversely to the same cytokine.
Our results showed that IL-13 and TNF-α up-regulated involucrin levels in both normal and scleroderma keratinocytes, which is not consistent with a previous study that has shown that IL-13 significantly inhibits involucrin mRNA expression and TNF-α did not affect involucrin in epidermal keratinocytes [12]. The reason behind these discrepancies may be related to post-transcriptional and post-translational modifications, as well as the stability of the involucrin protein [13]. Recent studies suggest that protein concentrations correlate with the corresponding mRNA levels by only 20–40% and that mRNA abundances are only a weak surrogate for corresponding protein concentration [14].
Moreover, blocking Rho function results in inhibition of keratinocyte terminal differentiation and increased cell proliferation [15]. In our experiments, Y-27632 alone did not show any effect on the involucrin expression in both normal and scleroderma keratinocytes. A previous report has claimed that in suspension normal human epidermal keratinocytes, involucrin-positive cells were remarkably decreased in the presence of Y-27632 [15]. The reason for this inconsistency may be due to the difference between cell states. In our experiment, the cells were cultured adherently, not being trypsinized.
Furthermore, IFN-γ decreased involucrin in both normal and scleroderma keratinocytes when pretreated with Y-27632, suggesting that the Rho signalling pathway may play a role in IFN-γ-regulated involucrin expression. In addition, in normal keratinocytes, it seems that Rho does not play a role in IL-13-induced involucrin as IL-13 could still upregulate involucrin after Rho is inhibited. However, in scleroderma keratinocytes, IL-13 decreased involucrin after inhibiting the Rho signalling pathway, indicating that Rho may play an active role in the production of involucrin in scleroderma keratinocytes. This further confirms that different cellular phenotypes may respond differently to the same cytokine.
Conclusions
These findings suggest that in scleroderma, the epidermal differentiation is delayed, which may be related to inflammatory cytokines, such as IL-4, IL-6, IL-10, TGF-β1, ET-1, IFN-γ, VEGF, PDGF-BB, and Rho signalling pathway. Targeting these factors may change the differentiation of scleroderma keratinocytes.








