Introduction
Hepatitis C virus (HCV) infection is a global health problem and a major risk factor for cirrhosis, hepatocellular carcinoma, hepatic decompensation, and liver transplantation. Notably, the World Health Organization has advocated eliminating HCV as a public health goal by 2030 [1]. The global prevalence of HCV declined from 0.9% to 0.7% between 2015 and 2020 driven by expanding access to point of care diagnostics (i.e. HCV RNA testing), effective strategies in HCV transmission control and wide availability of highly effective direct acting antiviral therapies [2]. The East Mediterranean and European areas had the greatest infection rates, accounting for 470,000 (62.5/100,000) and 300,000 (61.8/100,000) cases, respectively [3]. Around 4.61% of Egyptians tested positive for HCV antibodies, and 76.5% of these patients had viremia [4].
According to a global epidemiological study, exposure to infectious organisms was found to be a factor in 2 million (16%) out of 12.7 million newly diagnosed cancer cases. The prevalence was higher in developing countries [5].
Despite the fact that HCV is a hepatotropic virus, numerous studies have revealed that it can infect organs and tissues besides the liver, such as the adrenal gland, pancreatic tissues, heart and peripheral blood cells [6, 7]. Additionally, extrahepatic cancers such as pancreatic and lung cancers have been linked to HCV infection [8, 9].
However, the association between HCV infection and extrahepatic malignancies among the Egyptian population has not been investigated enough. This study aimed to test the association between HCV seropositivity and development of lung cancer, colorectal cancer, pancreatic cancer and breast cancer in the Egyptian population and whether HCV seropositivity has an impact on cancer outcome in those patients.
Material and methods
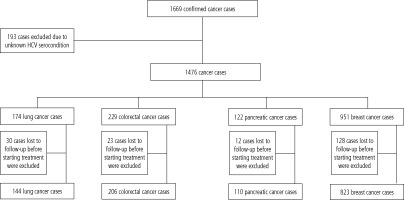
This study is a retrospective case-control study. 1669 patients diagnosed with lung, colorectal, pancreatic and breast cancers were identified in the hospital’s medical records as being admitted to the oncology department, Beni Suef University hospital in the period from January 1st 2017 to December 31st 2021.
The following information was collected from the hospital medical record system for all confirmed lung, colorectal, pancreatic and breast cancer patients: age, sex, initial tumor stage information (TNM), tumor grade, tumor histopathological type, anti-HCV serological status at diagnosis, survival duration, the date and status at the last hospital visit and other related data.
Exclusion from the study was applied for 193 cancer cases because of their unknown HCV serocondition.
The remaining 1476 cases were assigned to four subgroups according to their cancer type (lung: 174, colorectal: 229, pancreatic: 122 and breast: 951 cancer cases) (Fig. 1).
1550 age- and sex-matched controls were randomly selected from attendants to Beni Suef University hospital away from the oncology department throughout the same time period.
Subjects with HCV seropositivity were defined as those with positive HCV antibodies.
The difference in prevalence of HCV seropositivity between the cancer group and the control group was analyzed.
After establishment of HCV seroprevalence among eligible subjects, 193 cases were further excluded from cancer group as they were lost to follow up before starting cancer treatment.
The remaining 1283 cancer cases (lung: 114, colorectal: 206, pancreatic: 110 and breast: 823 cancer cases) were subdivided into HCV seropositive and seronegative subgroups and were compared regarding age, initial TNM staging, histological grading, histopathological type and cancer survival.
TNM staging [10]: The TNM stage is still the most important element in predicting survival.
Histologic grading [11]: Histologic grading is a simple and low-cost approach to assessing tumor behavior and prognosis.
Age [12]: Older age is a negative predictor of survival in solid cancer patients.
Collection of data and statistical analysis were carried out until 2022.
Statistical analysis
Data were analyzed using the software SPSS ver-sion 20 (IBM, Armonk, NY), then processed and tabulated and visually illustrated by charts and graphs.
The mean and standard deviation were calculated for continuous variables with a normal distribution. The chi-square (χ2) test for comparing the categorical data and independent t-test for comparing the quantitative data were performed where appropriate. The odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were measured to evaluate the association between HCV seropositivity and cancer development.
The hazard ratios (HRs) and corresponding 95% Cis were measured by the Cox regression analysis tool to evaluate the association between HCV seropositivity and survival among extrahepatic malignancies.
Survival duration analysis was done using the Kaplan-Meier survival analysis tool.
P values of less than 0.05 were considered significant.
Ethical consideration
The study was approved by the ethical committee of the Faculty of Medicine, Minia University. The subjects or their legal representatives were informed of the purpose of the study and its consequences with confirmation of confidentiality of data. The study protocol and conduction conform to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
This retrospective case control study included 1476 cancer patients who were categorized according to their cancer type and compared to 1550 age- and sex-matched controls for HCV seropositivity. From them, 1283 cases were tested for the relation between HCV seropositivity and survival.
There was no significant difference between the cancer group and control group regarding mean age and sex distribution (Table 1).
Table 1
Sex and age distributions of patients with extrahepatic malignancies
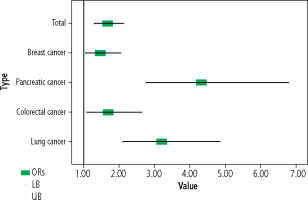
The percentage of HCV seropositive subjects was significantly higher in the total cancer group compared to that in the control group and in cancer types including lung, colorectal, pancreatic cancers and in female breast cancer patients compared to female controls (Table 2 and Fig. 2).
Table 2
HCV antibody seroprevalence among total cancer group and included types compared to the control group
There was no statistically significant difference between HCV seropositive and seronegative subjects regarding cancer histological grading, histopathological type and TNM staging except in colorectal cancer, where HCV seropositive subjects were significantly more distributed in late TNM stages (Table 3).
Table 3
Clinicopathological criteria of extrahepatic malignancies in relation to HCV serocondition
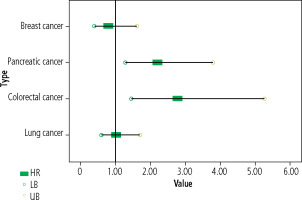
There was significantly lower survival among HCV seropositive subjects compared to seronegatives in colorectal and pancreatic cancers. In lung cancer HCV seropositive subjects showed non-significantly lower survival while in breast cancer seropositives the survival was non-significantly higher compared to seronegatives (Table 4 and Fig. 3).
Table 4
Association between HCV infection and survival among subjects in cancer group
Fig. 3
Forest plot shows the association between HCV infection and survival among subjects in cancer groups
There was a significant decrease in the mean survival duration among HCV seropositive subjects compared to HCV seronegatives in colorectal and pancreatic cancers. In lung and breast cancers, the difference was not significant (Table 5 and Fig. 4).
Table 5
Mean survival duration (in months) in HCV seropositive and seronegative subjects in included cancer types
Discussion
Hepatitis C virus is one of the most common blood-borne pathogens [13]. Cancer is one of the top four causes of human mortality [14]. Many studies have reported that chronic HCV infection has been linked to an increased risk of extra-hepatic cancers [15, 16].
Our study targeted HCV antibody seroprevalence among extrahepatic malignancies including lung, colorectal, pancreatic and breast cancers to test whether HCV seropositivity influences cancer survival in the Egyptian population.
In this study, HCV seroprevalence was significantly higher among the total cancer group compared to the control group (11.6% vs. 7.3%, OR = 1.67, 95% CI: 1.30-2.14, p < 0.001), suggesting a significant association between HCV seropositivity and cancer development.
These data coincide with Mahale et al., who reported that HCV prevalence was significantly higher in cancer cases than in non-cancer controls (OR = 1.32, 95% CI: 1.22-1.42, p < 0.0001) [17]. Nyberg et al. found that the non-hepatic cancer burden was significantly higher in HCV patients than in non-HCV (risk ratio = 1.83, 95% CI: 1.74-1.93) [18].
In the current study, HCV seropositivity was higher among patients with lung cancer compared to the control group (20.1% vs. 7.3%, OR = 3.20, 95% CI: 2.11-4.86, p < 0.001).
These results are in accordance with Lam et al., who observed that HCV infection was associated with an increased risk of lung cancer (aIRR = 1.3, 95% CI: 1.2-1.5) [19]. Ponvilawan et al. reported that chronic HCV infection was significantly associated with an increased risk of lung cancer with the pooled risk ratio of 1.94 (95% CI: 1.56-2.42) [20].
In contrast to our results, Kamiza et al. also reported that HCV infection was not linked to increased risk of lung cancer development (HR = 0.87, 95% CI: 0.62-1.21) [21].
This study found that subjects with colorectal cancer showed a significantly higher percentage of HCV seropositivity compared to controls (11.8% vs. 7.3%, OR = 1.70, 95% CI: 1.09-2.65, p = 0.025).
These results coincide with those of Su et al., who found that the prevalence of chronic HCV infection was higher in colorectal cancer cases than in controls (OR = 1.16, 95% CI: 1.08-1.24, p < 0.001) [22]. Similarly, Darvishian et al. reported that the risk of colorectal cancer was significantly elevated among those with HCV infection compared to others without (HR = 2.99; 95% CI: 2.55-3.51) [23].
In contrast to our results, Mahale et al. reported that there was no significant association between HCV infection and cancer colon (OR = 1.02, 95% CI: 0.93-1.13) [17].
In this study, HCV seropositivity was significantly higher among subjects with pancreatic cancer compared to controls (25.4% vs. 7.3%, OR = 4.33, 95% CI: 2.76-6.80, p < 0.001).
These results agree with Abdelrehim et al., who found that HCV infection was significantly associated with pancreatic cancer (OR = 3.9, 95% CI: 1.13-13.40) as one of some potentially modifiable factors including tobacco smoking, diabetes and cholecystitis [24]. Darvishian et al. concluded that people with HCV infection were at increased risk of pancreatic cancer compared to uninfected individuals (HR = 2.79, 95% CI: 2.01-3.70) [23]. Lam et al. observed that HCV infection was associated with an increased risk of pancreatic cancer (aIRR = 2.0, 95% CI: 1.6-2.5) [19].
On the other hand, Liu et al. found that HCV seroprevalence was not higher in patients with pancreatic cancer compared to controls (0.75% vs. 0.48%, OR = 1.56, 95% CI: 0.67-3.61, p = 0.296) [25].
Women with breast cancer in this study had a significantly greater percentage of HCV seropositivity than women in the control group (8.1% vs. 5.7%, OR = 1.47, 95% CI: 1.05-2.05, p = 0.031).
These findings agree with those of Hussein et al. They discovered that among individuals with breast cancer there was a statistically larger percentage of HCV seropositive subjects than among those without the disease (21.7% vs. 10.3%, p = 0.0027) [26]. According to Cheng et al., HCV infection was substantially linked to the development of breast cancer in people under the age of 49 (HR = 2.19, 95% CI: 1.097-4.384) [27].
In contrast to our results, Wang et al. found that HCV infection was adversely correlated with breast cancer (HR = 0.87, 95% CI: 0.77-0.97) [28]. According to Liu et al., there was no difference in the prevalence of HCV between breast cancer patients and controls (0.5% vs. 0.65%, OR = 0.76, 95% CI: 0.48-1.21, p = 0.244) [25].
The current study showed a non-significant difference between HCV seropositive and seronegative subjects regarding TNM cancer staging, grading and histopathological types in lung, pancreatic and breast cancers.
These results coincide with those of Allison et al., who reported that the grade and stage of cancer in patients with chronic HCV were not remarkably different from controls [29]. Hussein et al. stated that antiHCV serology status did not affect the staging and grading of breast cancer [26].
In colorectal cancer, HCV seropositive subjects were significantly more distributed in late stages (90.9% vs. 65.8%, p = 0.016) and there was no significant difference regarding cancer grading and histopathological type.
This could be attributed to delayed presentation and late diagnosis of cancer in those seropositive patients.
Our results demonstrated that the survival duration in HCV seropositive subjects was significantly lower compared to seronegatives in the case of colorectal cancer (15 vs. 39.1 months, HR = 2.77, 95% CI:1.45-5.26, p = 0.002) and pancreatic cancer (7.3 vs. 15.1 months, HR = 2.20, 95% CI: 1.28-3.78, p = 0.004), while it was nonsignificantly lower in lung cancer (p = 0.927) and nonsignificantly higher in breast cancer (p = 0.513).
These results indicate that HCV seropositivity was associated with significant reduction in survival duration in patients with colorectal and pancreatic cancers.
Late TNM staging and older age may be considered in the causation of reduced survival in HCV seropositives in colorectal cancer, while in pancreatic cancer subjects HCV seropositivity is the only considered factor. Other possible risk factors such as co-morbidities and environmental factors should be examined in future studies.
These results coincide with Allison et al., who found that cancer mortality was significantly higher among HCV infected patients with rectal cancer (RR = 2.6, 95% CI: 2.5-2.7, p = 0.04) and pancreatic cancer (RR = 1.63, 95% CI: 1.6-1.7, p = 0.001) [29]. Hussein et al. reported that there was no statistically significant difference between HCV seropositive and seronegative subjects regarding survival in breast cancer (HR = 1.65, 95% CI: 0.74-3.21, p = 0.249) [26]. Lee et al. documented that HCV seropositives did not have a higher mortality from lung cancer than HCV seronegatives (HR = 1.08, 95% CI: 0.59-1.98) [30].
In contrast to our study, Lee et al. found that HCV seropositive subjects did not have a higher mortality from colon cancer than HCV seronegatives (HR = 1.65, 95% CI: 0.59-4.60) [30]. Allison et al. documented that the mortality from lung cancer was significantly lower in the HCV infected group (RR = 0.98, 95% CI: 0.97-0.98, p < 0.001); they also stated that breast cancer mortality in the HCV infected group was significantly lower than controls (RR = 0.42, 95% CI: 0.41-0.43, p = 0.02) [29].
The disparity between our results and those of other studies may be attributed to variations of the geographic areas, HCV endemic status, age, sex and race of included subjects in those studies. Liu et al. stated that the association of HCV infection with cancer incidence was particularly notable in geographical areas of endemic HCV infection [25].
Conclusions
Our study suggested a significant positive association between HCV seropositivity and development of lung, colorectal, pancreatic and breast cancers. HCV seropositivity had no impact on cancer grading and histopathological type but was associated with reduced survival in colorectal and pancreatic cancers. Other environmental factors and co-morbidities that may reduce survival in these two cancer types should be considered and examined in further studies.
Limitations of this study
Our study was a retrospective case control one and data were taken from available patients’ medical records. We could not definitively link HCV seropositivity to the examined extrahepatic malignancies because even though age and sex were matched, other factors such as environmental factors, occupational factors, and associated comorbidities could not be analyzed. Our sample was uni-centric and relatively small.