INTRODUCTION
In the primary immunodeficiency spectrum, the most common symptomatic type is common variable immunodeficiency (CVID) [1]. Diagnostic criteria include serum immunoglobulin G (IgG) at least 2 standard deviations below age-appropriate reference levels in individuals over 4 years of age, low serum immunoglobulin A (IgA) and/or immunoglobulin M (IgM), impaired or absent accompanying specific antibody production, and exclusion of other causes of hypogammaglobulinaemia [2].
Although recurrent-persistent infectious complications are more common in patients with CVID, non-infectious complications such as interstitial lung disease, enteropathy, autoimmunity, allergic diseases, lymphoproliferation, and malignancy can also be seen [2]. The molecular defects that lead to CVID are still unknown; however, it has been reported to be caused by errors in the innate and acquired immune systems [3, 4].
About 90% of patients with CVID have normal B-cell levels. Therefore, differentiation errors during the final stage of the B cell may be associated with major causes of the disease [5–8]. The defect in antibody production in these patients suggests that there is a defect of transformation process of B cells into plasma and memory cells [5]. The numbers of plasma cells, class-switched memory B cells (cSMBCs) (CD19+CD27+IgD−), and IgM memory B cells (CD19+CD27+) are reduced in patients with CVID [9–11].
Progression of coronavirus disease-2019 (COVID-19) is associated with the host’s immune response to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) at the time of first encounter [12]. As well as T cells and natural killer (NK) cells, B cells also play a critical role in establishing a memory response to clear cytopathic viruses and preventing re-infection [13].
AIM
However, the relationship of B cells and antibody production to SARS-CoV-2 infection remains unclear. In this study, we investigated the association of cSMBCs with the clinical course of COVID-19 in patients with CVID.
MATERIAL AND METHODS
STUDY DESIGN
This study was carried out retrospectively at Necmettin Erbakan University Faculty of Medicine, Adult Allergy, and Immunology Clinic. The Local Ethics Committee approved the study protocol (decision no. 2022/3889), which complied with tenets of the Declaration of Helsinki (1975). Due to the retrospective data-scanning method of the study, it was optional to obtain informed consent after Ethics Committee approval. Therefore, informed consent was not obtained from the patients. Patients with CVID followed in our clinic were screened for COVID-19 infection. Between March 2020 and June 2022, the clinical features, basal cSMBC level, and IgG, IgM, IgA, and CD19+ B cell levels of CVID patients with polymerase chain reaction (PCR)-positive COVID-19 disease were evaluated. Among 62 adult CVID patients, 24 patients with COVID-19 were included in the study. The patients were classified according to disease severity based on the clinical features specified by the National Institutes of Health and data in the hospital archives [14].
Mild disease: Presence of any symptoms of COVID-19, such as fever, sore throat, weakness, myalgia, headache, cough, loss of taste and smell, nausea, vomiting, or diarrhoea, without abnormality in lung imaging, excluding shortness of breath.
Moderate disease: Clinical signs or imaging of lower airway involvement but room air oxygen saturation (SpO2) ≥ 94% at sea level.
Severe disease: Lung infiltrates > 50% or respiratory rate > 30/min, SpO2 < 94%, PaO2/FiO2 < 300 mmHg in room air at sea level.
COVID-19 symptoms, hospitalisation duration, the results of PCR tests performed at 48-hour intervals on nasal and oropharyngeal swabs during hospitalisation, and positivity duration were recorded.
DATA COLLECTION
Demographic information, medical history, and laboratory values of patients were obtained from the local medical archive and recorded in an electronic database. Symptoms, hospitalisation history, vital signs (to determine disease severity), oxygen saturation, radiological findings, serum IgG, IgM, IgA levels, number of B lymphocyte subgroups, and basal cSMBC levels were recorded. The values of cSMBC and B lymphocyte subgroups were taken from the records of initial diagnosis of CVID. Immunoglobulin levels were measured on the first day of outpatient or inpatient follow-up during the diagnosis of COVID-19 disease before receiving treatment. The relationships among COVID-19 symptom duration, duration of PCR positivity, hospitalisation duration, disease severity, mortality, serum immunoglobulin, and basal cSMBC levels were evaluated.
SARS-COV-2 RNA ISOLATION
SARS-CoV-2 RNA was detected by reverse transcription-PCR (RT-PCR) from simultaneous oropharyngeal and nasopharyngeal swabs using a VNAT solution (Bioaxen, Istanbul, Turkey) with the Bio-Speedy SARS-CoV-2 RT-qPCR kit (Bioaxen) on a Rotor-Gene Q instrument (Qiagen, Antwerp, Belgium). Results were analysed using Rotor-Gene Q software.
SERUM IMMUNOGLOBULIN MEASUREMENTS
Serum immunoglobulin levels were measured using a nephelometric method (BN II System; Siemens, Erlangen, Germany). When the diagnosis of COVID-19 infection was made, the serum IgG, IgM, and IgA values measured just before receiving intravenous immunoglobulin replacement therapy (IgRT) were recorded on the first day of follow-up.
FLOW CYTOMETRIC ANALYSIS
Peripheral blood samples were taken at the time of diagnosis of CVID and tested in anticoagulant tubes with EDTA within 6 h. B-cell subset numbers (cells/ml) were counted using multicolour flow cytometry. Surface markers were detected using human monoclonal anti-CD27 phycoerythrin (PE), anti-CD19 allophycocyanin (APC), and anti-IgD fluorescein isothiocyanate (FITC) (all BD Biosciences, Erembodegem, Belgium) antibodies. Cells were analysed using a BD FACS Canto II flow cytometer (BD Biosciences). The B-cell subgroups were naive B cells (CD19+ CD27−IgD+) cSMBCs (CD19+CD27+IgD−) and non-switched memory B cells (CD19+CD27+IgD+). None of the patients were receiving any immunosuppressive therapy, including steroids, at the time of flow cytometric analysis.
STATISTICAL ANALYSIS
Continuous variables are given as medians with interquartile range (IQR), and categorical variables as numbers and percentages for each category. The Mann-Whitney U test was used to evaluate continuous data according to the disease severity and cSMBC level. Spearman’s correlation was used to assess the association between cSMBC level and PCR positivity duration. SPSS statistical software (ver. 22.0; SPSS Inc., Chicago, IL, USA) was used in the analysis. P-values < 0.05 were considered indicative of statistical significance.
RESULTS
All patients in the study were infected by the ancestral Wuhan-like strain, no variant was detected. Also, patients had COVID-19 disease before their first vaccination. The median age of 24 patients was 48 years (IQR: 32–58), and 62.5% of them were female. The patients were hospitalised and followed up, and 3 (13%) were followed in the intensive care unit. One-third of the patients had severe disease. The clinical courses of the patients are summarised in Table 1. Three of the 8 patients in the severe group (37.5%; aged 34, 41, and 65 years) required intensive care, all of whom died during follow-up. There were comorbidities (asthma, ulcerative colitis, liver cirrhosis) in the patients who died.
TABLE 1
Clinical course of coronavirus disease-2019
All patients were undergoing Ig replacement therapy (IgRT) every 3 weeks. However, regardless of the time of the last routine IgRT dose, we additionally repeated IgRT at the time of diagnosis of COVID-19. The median time between the last IgRT and the onset of symptoms was 13 days (min.–max.: 3-20).
CD19 levels were similar between the mild-moderate and severe disease groups, but the number of cSMBCs was found to be lower in the severe group (p = 0.018). IgG levels were significantly lower, and patients were significantly older, in the severe disease group (Table 2). To investigate the age-related changes in the rate of cSMBCs, patients were divided into 2 groups under 50 years old and over. Median cSMBC was 3.4% (IQR: 1.8–9.7) in patients under 50 years of age and 4.6% (IQR: 3.7–5.2) in patients over 50 years of age. No significant difference was found between age groups (p = 0.379).
TABLE 2
Immunoglobulins, CD19 and class-switched memory B-cell levels according to coronavirus disease-2019 severity
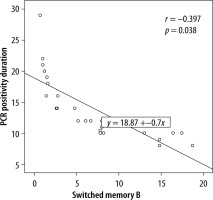
The cSMBC level was in the normal range (9.2–18.9 cells/ml) in 13 patients and low in 11 patients; the latter patients had longer durations of hospitalisation, symptoms, and SARS-CoV-2 PCR positivity (Table 3). The cSMBC level was significantly negatively correlated with the SARS-CoV-2 PCR positivity duration (r = 0.397, p = 0.038) (Figure 1).
TABLE 3
Class-switched memory B-cell levels and coronavirus disease-2019 clinical findings in patients with common variable immunode-ficiency
DISCUSSION
In this study, a low cSMBC level was associated with severe disease, increased mortality, and prolonged hospitalisation, symptom duration, and PCR positivity. Decreased total B and cSMBC numbers in the normal population are known independent risk factors for COVID-19-related mortality and increased disease severity [15]. In a multivariate analysis of indicators of COVID-19 disease severity, impaired activation of monocytes and neutrophils, depletion of active cSMBCs, and severe depletion of NK cells as the most prominent early-onset immunological markers of severe COVID-19 disease were found [16]. However, there have been no studies evaluating the relationship between cSMBC level and the clinical course and severity of COVID-19 in patients with CVID.
The COVID-19 pandemic has affected more than 600 million people and caused the death over 6 million people [17]. The high contagiousness of SARS-CoV-2 and rapid increase in the number of affected patients is associated with the lack of an immune response to exposure to viral antigens [18]. COVID-19 may follow a course ranging from asymptomatic to acute respiratory distress syndrome and multi-organ failure, and it may result in death [19, 20].
CVID is a type of primary immunodeficiency in which T- and B-cell abnormalities accompany hypogammaglobulinaemia. A meta-analysis of 14 studies evaluated data from 68 patients with CVID and COVID-19. In that study, approximately 29% of CVID patients had moderate to severe disease (needing oxygen support), and 10% were classified as critically ill (requiring intubation); the COVID-19-related mortality rate was 13% [21]. In this study, we found that one-third of patients with CVID had severe COVID-19 disease, 37.5% of patients with severe disease, and 12.5% of all patients died.
The mortality rate of patients with CVID is significantly increased compared to the general population [21, 22]. The lack of systematic evaluations in most cohort studies suggests that the mortality rate may be overstated, given that mild or asymptomatic cases may have been excluded [23]. An impaired B lymphocyte response and IL-6 deficiency in patients with CVID may protect against macrophage hyperactivation and cytokine storm [24]. Although a lower mortality rate was reported in a later large-scale systematic study, it was higher than in the normal population [25].
T and B cells are essential for eliminating viral infections. T cells exert their effects via the chemokines and cytokines released from antigen-presenting cells, and B cells produce neutralising antibodies [26]. After B-cell activation, specific memory B cells differentiate into high-affinity plasma cells during recurrent infection. Memory B cells and T cells are major components of the virus-specific protective immune response [27].
The B cell begins to develop in the bone marrow and completes its development in the peripheral tissue. Immature B cells differentiate into marginal zone B cells or naive peripheral follicular B cells. Marginal zone B cells transform into IgM memory B cells. Follicular B cells differentiate into cSMBCs and antibody-secreting plasma cells in the germinal centres [9, 28]. Memory B cells are recognised by the CD27 surface marker. B cells with IgM and IgD on their surface are non-switched memory B cells; cSMBCs are those that have IgG, IgA, or IgE on their surface but lack IgM and IgD [5]. Proper development of germinal centres is required for the production of cSMBCs in secondary lymphoid organs. Germinal centre impairment is related to a reduced number of cSMBCs [23]. Memory B cells can survive for years after encountering antigens. Exposure to antigens or environmental signals may induce their differentiation into antibody-secreting plasma cells [5].
Patients with COVID-19 pneumonia and more memory B cells show accelerated recovery [29]. There is a negative correlation between COVID-19 symptom duration during convalescence and the cSMBC level [30]. SARS-CoV-2–specific memory B cells are formed after about 1 month; this early protective immune response begins before specific memory B cell formation. This may be associated with enhanced cross-reactive B- and T-cell responses resulting from previous exposure to coronaviruses [31].
Patients with CVID who have a low cSMBC level have more frequent recurrent respiratory tract infections and bronchiectasis. Such patients require more special care services, and the cSMBC level could have prognostic value [32]. In this study, a small number of cSMBCs were associated with mortality, severe disease, and greater durations of hospitalisation, symptoms, and PCR positivity. Also, the number of cSMBCs was significantly negatively associated with the duration of PCR positivity.
This study had some limitations. First, it was a cross-sectional, retrospective study. Second, we did not enumerate memory B cells specific for SARS-CoV-2. Third, a larger number of patients would have increased the statistical power.
To the best of our knowledge, this is the first study on the relationship between the clinical course, severity, and mortality of COVID-19 and the cSMBC level in patients with CVID. The findings provide insight into the immune response to SARS-CoV-2 and other viruses.
CONCLUSIONS
The durations of SARS-COV-2 PCR positivity, hospitalisation, and symptoms were more severe in patients with CVID who had a low cSMBC level. CVID patients with severe COVID-19 had lower cSMBC levels. In patients with prolonged SARS-CoV-2 PCR positivity, this was associated with lower cSMBC levels. Therefore, measurement of the basal cSMBC levels in patients with CVID may enable early identification of those at risk of severe COVID-19. In such patients, timely administration of aggressive treatments (e.g. high-dose intravenous immunoglobulin and antivirals) would reduce the morbidity and mortality rates.