Introduction
Thymectomy is the commonest anterior mediastinum surgical treatment, and it is a proven procedure for both malignant and benign thymus disorders. Thymic gland removal is now a well-recognized surgical method in myasthenia gravis (MG) management and, at the same time, thymectomy is the primary procedure for thymic tumours, especially in the early stages [1, 2].
MG is a neuromuscular illness described by fluctuating and fatigable weakness in different groups of muscles. It happens as a result of autoantibody production targeted against components of the neuromuscular junction [3]. The medical MG management involves immunosuppressive therapy and symptomatic therapy use (anticholinesterase). Since Blalock has shown a symptomatic improvement in an MG patient who went through thymectomy for thymoma in the early twentieth century and later reported the same outcomes in MG patients who went through thymectomy, this procedure has played an important role in establishing thymectomy as a widely recognized treatment approach in nonthymomatous MG management [1]. At the same time, thymectomy is the cornerstone of thymic tumour treatment [2].
Other surgical methods for thymectomy have been documented, which range from open (mostly transsternal) to minimally invasive methods (thoracoscopic or transcervical). Still, median sternotomy for several years has been deemed as the standard procedure. Certainly, the advancement of minimally invasive surgery has contributed to a rise in thymectomy acceptance, particularly for benign conditions. However, thymomas continue to be a source of concern.
The introduction of robotic-assisted surgical systems has been another step forward in the development and evolution of minimally invasive strategies with a clear technical benefit over standard video-assisted thoracoscopy, especially for surgical applications in remote-to-reach or narrow anatomical regions such as the mediastinum [4]. Table I shows the distribution of the largest series consisting of 449 robotic thymectomies at the Charite in Berlin. Robotic thymectomy has also been conducted on children as young as 4 years old and on subjects as old as 86 years old [5].
Table I
Indications for robotic thymectomy [5]
| S. no. | Indications | No. of cases |
|---|---|---|
| 1 | MG | 397 |
| 2 | Thymoma | 64 |
| 3 | Thymoma + MG | 53 |
| 4 | Parathyroid gland | 7 |
| 5 | Others | 29 |
History of robotic thymectomy
In 1939, Blalock et al. [6] were among the first to mention a significant improvement in symptoms after thymectomy in an MG and a cystic thymic tumour patient. Since then, numerous other studies have been published, all of which show that thymectomy has a favourable outcome in nonthymomatous myasthenia gravis. However, the true advantage of this technique remains in doubt, given the lack of any formal proof [7].
An in-depth assessment of retrospective studies was published in 2000 [8], and most findings showed a positive outcome to thymectomy with regards to illness improvement or remission rates. But the researchers were unable to draw definitive results due to numerous methodological shortcomings. The Thymectomy Trial in Non-Thymomatous Myasthenia Gravis Patients Receiving Prednisone Therapy (MGTX) was a big breakthrough in the thymectomy role for non-thymomatous myasthenia gravis patients in the year 2016 [9]. The goal of this large, single-blind, randomized experiment was to find out if extended transsternal thymectomy coupled with a standardized prednisone treatment was better than standardized prednisone treatment alone after 3 years. A total of 126 subjects with generalized nonthymomatous myasthenia gravis from 36 institutions were randomized to 2 treatment arms based on strict criteria for inclusion (age 18–65 years, Myasthenia Gravis Foundation of America (MGFA) clinical class II to IV illness, positivity for acetylcholine-receptor (AChR) antibody, and duration of disease < 5 years). The study findings revealed unequivocally that thymectomy improved clinical outcomes and reduced the prednisone therapy need in subjects with generalized nonthymomatous myasthenia gravis [10].
Surgical techniques for thymectomy have progressed over time, with the goal of lowering surgical morbidity and increasing such procedures’ acceptance for benign illnesses, particularly among younger subjects. Subxiphoid, transcervical, video-assisted thoracoscopic surgery (VATS) and robot-assisted (robot-assisted thoracic surgery – RATS) thymectomy are examples of minimally invasive methods [11]. Several meta-analyses and authors have shown that a minimally invasive approach to thymectomy is related to superior surgical results and fewer complications in surgery compared to transsternal open thymectomy. The access with minimally invasive surgery VATS or RATS access in its various variants allows the extent of mediastinal fat resection due to the possibility of ectopic thymic foci in the mediastinum determining the long-term outcome in the group of patients operated on for myasthenia gravis [12]. With this background, the present review of the literature study focuses on describing and delineating the techniques, advantages, outcomes, and future perspectives of robotic thymectomy.
Robotic system
The da Vinci (Intuitive Surgical, Inc) robotic system is the most widely used these days. A console where surgeons sit during the operation, a cart that holds the interacting arms of the robot, as well as a magnified vision system are all part of the system. The surgeon operates at a console that is away from the operation table. The console is linked to a video system, which provides the surgeon with a HD (high definition), 3D (three-dimensional) view inside the body of the subject and to the robotic cart via binoculars located in the upper section of the console. The system translates the movements of the surgeon’s hand into smaller, identical, and more precise instrument movements inside the subject’s chest (Figure 1). Particular benefits of robotic surgery with the da Vinci system are as listed in Table II.
Figure 1
Components of the robotic system. A – Surgeon’s operative console, B – subject-side cart with interactive arms of robot, C – vision system
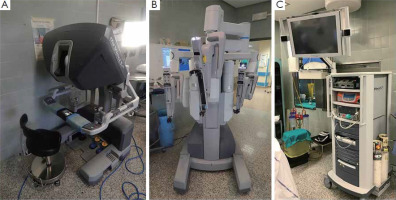
Table II
Technical benefits of robotic surgery with the da Vinci system
Motivation for robotic thymectomy
Robotic thymectomy may be deemed to be evolved from the VATS method. Indeed, the high-resolution 3D vision of the operating area, reduction in hand tremor and robotic arms’ articulation are distinct benefits of robot-assisted thymectomy compared to VATS thymectomy, particularly in difficult-to-reach or narrow anatomical locations such as the mediastinum. The researchers in a few trials that compared robot-assisted thymectomy technique to VATS noted that the former, i.e., RATS, is safe as well as feasible and has surgical benefits over the latter, i.e., VATS [13, 14]. Furthermore, Rückert et al. found that myasthenia gravis subjects treated using the robotic method had a better prognosis than those treated with VATS, which can be attributed to the superior mediastinal dissection obtained with robot-assisted thymectomy [13]. RATS thymectomy, on the other hand, has several drawbacks. Firstly, RATS is pricier compared to VATS thymectomy, with the majority of the cost going for the robotic system’s acquisition and disposable materials as well as its annual maintenance. Secondly, tactile feedback is lacking, which could put delicate anatomical components at risk of damage. However, the enhanced 3D view obtained by the robotic console, as well as better dexterity of robotic arms, appears to compensate amply for this. Finally, the surgeon who is operating is unscrubbed and positioned away from the subject; as a result, if there are any intra-operative difficulties that necessitate emergency conversion to sternotomy, another surgeon must remain sterile near to the subject [15–17].
Patient selection and preparation
During the preoperative examination, it is critical to determine whether any symptoms or clinical indicators of myasthenia gravis exist, and the serum titre of antibodies against anti-AChR should be evaluated [18]. If negative, anti-MuSK (muscle-specific receptor tyrosine kinase) antibodies ought to be analysed; there is proof that a positive serum titre of anti-MuSK Ab predicts a smaller thymectomy effect on symptoms of myasthenia gravis [18].
Furthermore, neurological evaluation must always be conducted before the surgical procedure to assess the presence of significant or active MG symptoms or to optimize the medical therapy. Especially, the corticosteroid levels must be reduced before surgery. Pre-operative intravenous immunoglobulin administration or plasmapheresis might reduce the post-operative respiratory failure risk, especially in subjects with symptoms that are partially controlled [19, 20]. Although there is no gold standard for surgery timing, it appears that removing the thymic gland early might boost the rate of remission [20]. In nonthymomatous myasthenia gravis, age beyond fifty years and antibody negative illness are relative contraindications to thymectomy [21, 22].
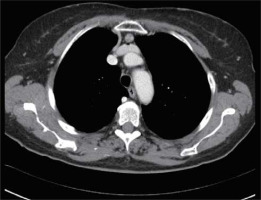
In addition, a contrast-enhanced computed tomography chest scan must be performed on all subjects for evaluation (Figure 2). PET-CT and/or magnetic resonance scan can also be conducted in the case of thymoma suspicion or to differentiate a small thymoma from thymic hyperplasia. Pre-operative X-rays of the chest must be carried out to check for extensive adhesions symptoms associated with the thoracic surgical operation or prior pleuritis, which might make a robotic approach impossible. Electrocardiogram, full examination of blood and pulmonary function tests must all be included in the functional evaluation.
The subject is ventilated through a double-lumen endotracheal tube during the procedure, which is conducted under general anaesthesia. A pulse oximeter, arterial line, urine output and ECG are all used to monitor subjects during the process. A bean bag is used to position the subject at a 30° angle, either right or left side up (depending on the operation side). The left arm is parallel to the bed in left-sided procedures, whereas the right arm is oriented along the body for exposing the axillary region (opposite for right-sided procedures). The surgeon console is placed away from the subject in the operation room, whereas the video column is placed at the bed bottom. The robotic arms cart is set at a 45° angle on the bed’s right side (opposite for the right-side approach) (Figure 3). The surgical field must always be wrapped for a possible median sternotomy due to the obvious emergency conversion risks.
RATS techniques
Since each surgeon seems to have a preferred method, both a left-sided and a right-sided method are possible. The procedure must be customized to the anatomy of the subject, so if necessary, a contralateral incision should be added. The key objective must be to attain a radical en-bloc resection of all thymic tissue from one to the next phrenic nerve and from the inferior poles of the thyroid gland to the diaphragm. The left-sided method has several advantages, including better contralateral phrenic nerve visualization, and is shielded inside its superior portion by the superior vena cava, and a greater distribution of the thymic gland and mediastinal fat to the left side and around the left phrenic nerve. On the other hand, surgeons who choose the right-sided method like the anatomical landmarks of the venous confluence and wider space (Figures 4 and 5) [23].
Figure 4
Surgical technique. A – Dissection along the phrenic nerve, B – dissection of the right inferior horn, with opening of the right pleura, C – division of the thymus from the pericardium, D – dissection towards the neck between the mammary vessels and the phrenic nerve (*, mammary vessels; ^, left subclavian vein; arrow, phrenic nerve)

Techniques of left-sided approach
At the left cardiophrenic angle level, the dissection begins inferiorly and extends along the phrenic nerve’s anterior border. The phrenic nerve must be isolated from all anterior mediastinal tissue and even fat. After that, detect and dissect the left inferior horn of the thymus from the pericardium. Later, separation of the retrosternal area from the thymic gland must be done until the right inferior horn and right mediastinal pleura are discovered. The thymus’ lower portion must be raised upward at this point, the left innominate vein must be located, and the dissection must proceed along the innominate vein’s border until the thymic veins are located, clipped and divided. The dissection must be continued up to the neck until the superior horns are detected and divided from the thyroid gland’s inferior portion. The thymus gland, anterior mediastinal and neck fatty tissues are resected en bloc, the medial port incision is enlarged slightly to 2 fingerbreadths, and the specimen must then be placed in an Endobag and removed. A 28F drain should be inserted through the medial port after haemostasis, the lung should be inflated, and other wounds should be closed. In the operation room, the subject should be extubated and then transferred to the ward.
Right-sided approach
Starting at the cardiophrenic angle and continuing upwards, the mediastinal pleura should be incised just anterior and medial to the right phrenic nerve. The superior vena cava and the nerve should be isolated from all anterior mediastinal tissue. The retrosternal parietal pleura ought to be then medially and parallelly opened to the right internal mammary vessels. The dissection of mediastinal tissue from the sternum anteriorly and pericardium posteriorly is done until the left brachiocephalic vein is detected. The veins of the thymus are located, clipped, and dissected. The superior horns then are detected and separated from the thyroid gland. The opening of the left pleura must next be done, and after identifying the left phrenic nerve, the thymus dissection is completed and the specimen extracted.
Refinements and modifications of RATS technique
The typical location of 3 trocars between the 3rd and 5th intercostal space and between the midaxillary and midclavicular lines might well be altered owing to usage of a 0-degree optic and additional trocars [1, 17, 24] Robotic aid, on the other hand, reduces the necessity for bilateral accessibility in more complex circumstances. The use of 5-mm instruments might be done for performing robotic thymectomy. New equipment with a movable tip for suction or dissection (vessel sealer) enables greater mediastinal dissection precision. Cardiac scissors seem to be highly precise and allow complete en bloc thymectomy for anatomical variations such as the encasement of the left phrenic nerve by thymic tissue. The proponents of the left-sided method emphasize superior visualization of a larger left-sided thymus, especially in complex overgrowth cases of the phrenic nerve (which occasionally occurs on the left side but never on right side virtually) as the key causes for obtaining complete thymectomy [23, 25–27]. Surgeons preferring easy conditions ensured by more space and the superior vena cava landmark should use the right-sided approach [24, 28, 29]. They state that trocar injuries can be prevented when coming from the right side [17]. The usage of no or continuous CO2 insufflation, monopolar hook belongs to robotic thymectomy variations [1].
Results
RATS thymectomy appears to have an outstanding safety profile, with a rate of morbidity ranging from 1.6% to 7.2% and no perioperative death in any of the investigations. Chylothorax, bleeding and myasthenic crises are the commonest consequences reported [23, 30–33]. Many single-centre case series have shown that RATS has superior post-operative results over open thymectomy regarding loss of blood, rate of morbidity and duration of stay in hospital [34–36]. Subjects undergoing thymectomy using minimally invasive techniques (mainly RATS) experienced fewer post-operative problems and spent less time in the hospital than subjects undergoing sternotomy, as per multi-centre research published in the French database EPITHOR. However, due to significant differences in characteristics of baseline subjects, no strong inferences concerning which procedure is superior to the other can be made [37]. Nevertheless, a recent systematic review compared the post-operative results after VATS or RATS thymectomy and found no substantial differences with regards to the duration of stay in the hospital, conversion to open surgery, and morbidity [38].
In terms of neurological results, non-surgical aspects such as thymoma presence (in comparison to thymic hyperplasia), the length of symptoms for more than 1 year, and older age are thought to reduce the thymectomy efficiency in alleviating MG symptoms [39]. On the other hand, complete thymic foci removal is the single most critical surgery dependent factor influencing the post-operative neurological outcomes [40, 41]. Sadly, retrospective investigations cannot usually estimate the extent of thymic tissue removal due to variances in surgical techniques and operative methods. In an effort to overcome this problem, the following definitions have been proposed: basic thymectomy involves thymic gland removal without any surrounding fat; extended thymectomy involves thymus removal with surrounding fatty tissue of the mediastinum and the neck [42]; lastly, the maximally extended thymectomy method which Jaretski proposed consisted in removal of the thymus with all mediastinal fat from the level of the upper poles of the thyroid gland to the diaphragm, as well as opening both the pleural cavities [40]. To achieve the highest rates of remission, the maximally extended procedure is suggested. Zielinski et al. compared the neurological outcomes of subjects who underwent thymectomy using 3 distinct procedures, demonstrating that the patient population treated with the most radical operational method had a higher rate of complete remission [43].
All authors reported satisfactory rates of complete remission ranging from 28% to 57% following robotic thymectomy [23, 30–33]. These findings are in accordance with the rates of complete remission obtained by transsternal thymectomy and vary from 15.8% to 60% [44]. Another neurological outcome measure is the proportion of subjects who have an improvement in symptoms of myasthenia gravis, as described by MGFA postintervention status classification, which ranged from 77 to 87.5 per cent in robotic thymectomy sequences [30–33]. Again, these findings are comparable to those obtained by transsternal thymectomy, which resulted in rates of palliation ranging from 79% to 86% (described as minor symptoms on no medication or symptom-free on medication) [30]. The outcomes of major series of robotic thymectomies are listed in Table III [45–50].
Table III
Outcomes of major series of robotic thymectomies
| Author | No. pts | Thymoma/nonthymoma | MG | OC (%) | OT [min] | Morbidity (%) | POS [days] | FU [months] | RR (%) | Thymoma 5-year survival (%) | MG remission (CSR, PR) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cakar et al. [45] | 9 | 4/5 | 9 | 0 | 154.0* | 11.1 | 13.0** | – | – | 100 I | |
| Augustin et al. [15] | 32 | 6/26 | NA | 3.1 | – | 6.2 | 6.0** | 25.0 | 0 | – | – |
| Rückert et al. [46] | 95 | 12/83 | 95 | 8.3 | 186.0* | 2.1 | – | 29.1 | 0 | – | > 40 CSR |
| Fleck et al. [47] | 18 | 0/18 | 18 | 5.5 | 175.0* | 33.3 | 4.2* | 18.0** | – | – | 55.5 |
| Goldstien and Yang [24] | 26 | 5/21 | 26 | 15.3 | 127.0* | 15.3 | 2.0* | 26.0* | – | – | 38.0 PR |
| Balduyck et al. [4] | 14 | 5/9 | 5 | 7.1 | 224.2* | 21.4 | 9.6* | 34.2* | – | – | – |
| Freeman et al. [30] | 75 | 0/75 | 75 | 0 | 113.0* | 6.6 | 2.2* | 45.0* | – | – | 53.3 |
| Rückert et al. [13] | 74 | 11/63 | 74 | 1.4 | 198.0* | 2.7 | – | 42.0* | – | – | 39.3 CSR |
| Mussi et al. [27] | 14 | 14/0 | 1 | 7.7 | 139.0* | 14.2 | 4.0* | 14.5** | 0 | 100 | 100 I |
| Melfi et al. [16] | 39 | 13/26 | 20 | 5.1 | 124.3* | 12.8 | 4.3* | 16.0** | 0 | – | 90.0 I |
| Marulli et al. [17] | 79 | 79/0 | 45 | 1.3 | 165.0* | 12.7 | 4.4* | 51.7* | 1.3 | 97 | – |
| Weksler et al. | 15 | 10/5 | 5 | 0 | 84.0* | 6.6 | 1.0** | – | – | – | – |
| Marulli et al. [31] | 100 | 8/92 | 100 | 0 | 120.0** | 6.0 | 3.0** | 67.0** | 0 | 100 | 28.5 CSR 87.5 I |
| Schneiter et al. [25] | 20 | 20/0 | 12 | 0 | – | 10.0 | 5.0** | 26.0** | 11.1 | 100 | – |
| Ye et al. [35] | 23 | 23/0 | 0 | 0 | 97.0* | 4.3 | 3.7* | 16.9* | 0 | 100 | – |
| Keijzers et al. [32] | 37 | 37/0 | 28 | 13.5 | 149.0* | 16.2 | 3.0** | 36.0** | 2.7 | 100 | 21.4% |
| Seong et al. [36] | 34 | 11/23 | 2 | – | 157.2* | 0 | 2.7* | 13.3* | 0 | – | – |
| Jun et al. [14] | 55 | 21/34 | NA | 0 | 139.8* | 10.9 | 7.2* | – | – | – | – |
| Huang et al. [48] | 23 | 23/0 | 1 | 0 | 85.2* | 4.3 | 3.6* | 24.8* | 0 | – | 100% I |
| Keijzers et al. [49] | 125 | 31/94 | 125 | 4 | 125** | 7.2 | 3.0** | 33.0** | 6.5 | – | 77% I |
| Marulli et al. [50] | 134 | 1134/0 | 70 | 8.9 | 140** | 17.1 | 4.0** | 48.0* | 0.7 | 100 | – |
Moreover, Kamel et al. reported from the experience of a single institution robotic thymectomy that it is safe and efficient in the management of thymic diseases, including thymomas, and an initial learning curve of 15 to 20 cases might be needed to attain technical proficiency [51]. Another study conducted by O’Sullivan et al. showed that as compared to open thymectomy, robotic thymectomy has numerous benefits, which include a shorter stay in hospital as well as reduced loss of blood. Though complication and death rates are comparable to VATS, robotic thymectomy is anticipated to provide technological benefits to the surgeon, such as Endowrist instrumentation, an in-built tremor filter and autonomous control of a tri-dimensional camera. The capital investment with yearly maintenance expenses remains a major disadvantage with the need for further economic assessment to estimate the long-term expense implications of VATS vs RATS [52].
Conclusions
The robotic method for thymectomy is nowadays regarded as a well-established and safe minimally invasive surgical procedure. In comparison to the transsternal method, RATS offers good neurological results and decreased surgical morbidity. Furthermore, RATS has shown positive long-term outcomes in nonthymomatous myasthenia subjects and positive oncological as well as surgical results in thymoma patients. Also, the improved vision and higher dexterity of instrument movements allow for complete and safe thymic tissue dissection, has not yet demonstrated scientific evidence in the literature to be superior to standard thoracoscopic methods. The access with minimally invasive surgery VATS or RATS access in its various variants allows the extent of mediastinal fat resection due to the possibility of ectopic thymic foci in the mediastinum determining the long-term outcome in the group of patients operated on for myasthenia gravis. As a result, robotic thymectomy is expected to become the standard thymectomy procedure in subjects suffering from early-stage thymomas as well as MG.
Future prospects
Sadly, due to the small number of subjects, different measures used to define neurological outcomes, variable inclusion criteria and differences in surgical approaches and operative techniques, it is impossible to compare neurological outcomes accurately between transsternal as well as minimally invasive thymectomy or thymectomy conducted utilizing distinct minimally invasive methods, such as subxiphoid, VATS and RATS. Furthermore, randomized controlled studies are not yet being undertaken to compare approaches to draw any definitive inferences. Hence, it was recommended to carry out better designed, multi-centre, randomized studies to arrive at definitive conclusions.