Introduction
Colon cancer is one of the most common malignant tumours and is at the forefront of tumour mortality in the world [1, 2]. In recent years, with the improvement of people’s living standards, the incidence of colon cancer has been increasing [2]. At the same time, the incidence of colon cancer is shifting to the right, and the incidence of right colon cancer has increased significantly. Surgical resection is one of the main treatments for colon cancer. Excision methods that minimize pain and ensure faster recovery have been investigated. Therefore, the treatment of colon cancer has undergone tremendous advances, from open surgery to minimally invasive approaches. Laparoscopic right colectomy (LRC) is a minimally invasive technique widely used in right-side colon cancer. However, conventional laparoscopy has some limitations, such as two-dimensional imaging, steep learning curve, magnified physiologic tremor, limited range of motion, and ergonomic discomfort.
In 2000, the robotic surgical system was officially used in clinical practice [3], and it was first applied to colon diseases in 2002 [4]. Robotic surgical systems have several advantages, such as a three-dimensional surgical view and increased flexibility and stability, which ensure the efficiency of the procedure for the benefit of the patient [5]. Consequently, robotic surgery has received increasing attention from surgeons, especially in robotic right colectomy (RRC). Previous studies have shown that robotic surgery is a safe and effective resection of colon cancer, and it is superior to laparoscopic surgery in many aspects, such as lower rate of conversion to laparotomy [6]. However, few studies have assessed the results of RRC, and its therapeutic effect remains controversial [7–9].
Aim
Therefore, the present meta-analysis aimed to evaluate the safety and feasibility of RRC and compare the short-term outcomes of RRC and LRC for colon cancer.
Material and methods
Search strategy
The study was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [10]. The PubMed, Web of Science, Embase, and Cochrane Library databases were searched for primary studies published in the last 5 years (January 2017–January 2022). For a more accurate search, the terms “colon cancer”, “right colectomy”, “robotic”, and “laparoscopic” were employed. All reference articles of the retrieved studies were reviewed to choose studies that better suit our criteria. No language restriction was applied.
Inclusion and exclusion criteria
Any studies that met the following criteria were considered: (1) all patients were diagnosed with colon cancer; (2) study compared RRC and LRC; and (3) the endpoints included postsurgical complications. If there were 2 or more articles by the same authors or research institutions, the most recent publication was selected.
Articles that met the following criteria were not considered: (1) those that included RRC or LRC; (2) studies in which data could not be extracted from the published results; and (3) article types that included abstracts presented at meetings, case reports, review articles, or letters.
Data extraction
Two authors independently collected data from each of the selected research papers, and disagreements were discussed before a final decision was made. The compiled information included author name, geographical region, year of publication, study period, type of study, surgical type, sample size, mean age, gender, body mass index (BMI), length of hospital stay, operation time, conversion to laparotomy, retrieved lymphatic nodes, estimated blood loss, overall postsurgical complications, ileus, anastomotic leakage, and wound infection.
Statistical analysis
RevMan 5.4 software (Nordic Cochrane Centre; Denmark) was used to analyse data. The risk ratio (RR) was employed to assess the dichotomous variables. If the I2 value was ≤ 50%, a fixed effects model was chosen, and if the value was > 50%, a random effects model was selected. To avoid publication bias, we used funnel plots. A value of p < 0.05 was considered statistically significant. In some publications, the mean values and standard deviation values were unavailable. Methods for determining these values from available median and range data have been described previously by Hozo et al. [11].
Results
Selected studies
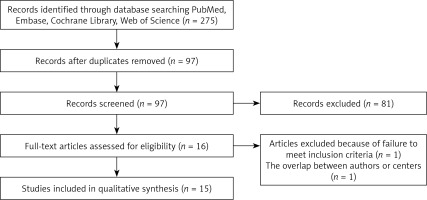
The study selection process is summarized in Figure 1. According to the retrieval strategy and data collection method, a total of 275 related studies were retrieved. By reading the titles and abstracts, we deleted 178 studies and initially included 97 studies. A total of 81 studies were eliminated due to the lack of right colon resection trial, of which 16 studies met the criteria. After reading the full text according to criteria and filtering for data integrity, 15 studies [12–26] with a total of 5142 patients (RRC group, 1116 patients; LRC group, 4036 patients) were eventually included in this meta-analysis.
Study characteristic
The characteristics of the individual trials are shown in Table I. A total of 1116 patients were included in the RRC group, whereas 4036 patients were included in the LRC group. The studies were from Italy (6 studies), the UK (one study), the USA (2 studies), Korea (one study), Denmark (2 studies), Turkey (one study), and China (2 studies).
Table I
Characteristics of the included studies
| Author | Nation | Year | Study period | Surgical type | Sample size | Gender (male/female) | Mean age [years] | BMI |
|---|---|---|---|---|---|---|---|---|
| Ceccarelli [12] | Italy | 2021 | 2014–2019 | RRC | 26 | 20/6 | 69.1 | 24.4 |
| LRC | 29 | 15/14 | 75 | 24.2 | ||||
| Dohrn [13] | Denmark | 2021 | 2015–2018 | RRC | 359 | 181/178 | 73.3 | 25.9 |
| LRC | 718 | 378/340 | 73.7 | 25.6 | ||||
| Haskins [14] | USA | 2018 | 2012–2014 | RRC | 89 | 49/40 | 68.9 | 29.3 |
| LRC | 2405 | 1129/1276 | 68.3 | 28.5 | ||||
| Khan [15] | UK | 2021 | 2007–2017 | RRC | 40 | 19/21 | 69 | 26 |
| LRC | 80 | 37/43 | 71 | 28 | ||||
| Mégevand [16] | Italy | 2019 | 2010–2015 | RRC | 50 | 28/22 | 70.3 | 26.2 |
| LRC | 50 | 24/26 | 69.6 | 25.2 | ||||
| Merola [17] | Italy | 2019 | 2012–2017 | RRC | 94 | 60/34 | 69.4 | 26.9 |
| LRC | 94 | 61/33 | 72.0 | 27.9 | ||||
| Park [18] | Korea | 2019 | 2009–2011 | RRC | 35 | 14/21 | 62.8 | 24.4 |
| LRC | 35 | 16/19 | 66.5 | 23.8 | ||||
| Rattenborg [19] | Denmark | 2021 | 2015–2018 | RRC | 22 | 9/13 | 71 | 25.5 |
| LRC | 40 | 15/25 | 73 | 25.5 | ||||
| Scotton [20] | Italy | 2018 | 1998–2017 | RRC | 30 | 108/98 | 70.1 | 26 |
| LRC | 160 | 80/80 | 70.3 | 25.6 | ||||
| Shi [21] | China | 2020 | 2016–2019 | RRC | 58 | 34/24 | NA | NA |
| LRC | 48 | 27/21 | NA | NA | ||||
| Sorgato [22] | Italy | 2021 | 2018–2019 | RRC | 48 | 27/21 | 71 | 25.6 |
| LRC | 40 | 28/12 | 68 | 26.6 | ||||
| Spinoglio [23] | Italy | 2018 | 2005–2015 | RRC | 101 | 57/44 | 71.2 | 25.1 |
| LRC | 101 | 47/54 | 71.2 | 25.8 | ||||
| Widmar [24] | USA | 2017 | 2012–2014 | RRC | 119 | 64/55 | 68 | 28 |
| LRC | 163 | 83/80 | 64 | 29 | ||||
| Yozgatli [25] | Turkey | 2018 | 2015–2017 | RRC | 35 | 20/15 | 65 | 29 |
| LRC | 61 | 31/30 | 65 | 27 | ||||
| Zeng [26] | China | 2020 | 2018–2019 | RRC | 10 | 4/6 | 57.8 | 21.4 |
| LRC | 12 | 3/9 | 61.3 | 22.2 |
Study quality
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the studies. The total NOS score was 9, and papers with a score ≥ 6 were classified as methodologically sound studies. All articles included in this study rated between 6 and 9, indicating that the study quality was sufficient (Table II).
Table II
NOS quality of included studies
[i] REC – representativeness of the exposed cohort, SNEC – selection of the nonexposed cohort, AE – ascertainment of exposure, DO – demonstration that outcome of interest was not present at the start of study, SC – study controls for age and sex, AF – study controls for any additional factors, AO – assessment of outcome, FU – follow-up long enough for outcomes to occur, AFU – adequacy of follow-up of cohorts (≥90%). “1” means that the study is satisfied the item and “0” means the opposite situation.
Operation time
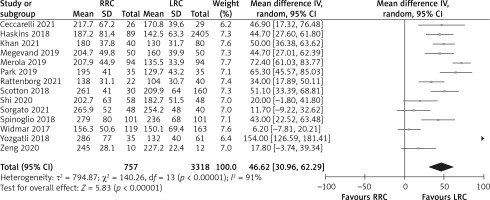
The operation time was reported in 14 studies, and 14 of the studies indicated that operation times were longer in the RRC group. Because of the high heterogeneity (I2 = 91%) among these studies, a random effects model was used for the meta-analysis. The results show that the RRC group had significantly longer operation times than the LRC group (MD = 46.62; 95% CI: 30.96 to 62.29; p < 0.001; Figure 2).
Conversion to laparotomy
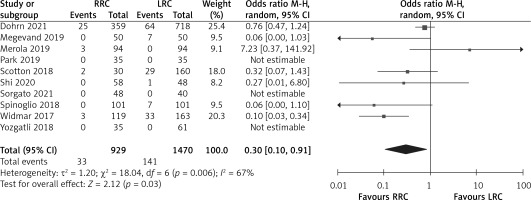
The rate of conversion to laparotomy was reported in 10 studies. The results of the meta-analysis showed that the RRC group had a significantly lower conversion to laparotomy than the LRC group (OR = 0.30, 95% CI: 0.10 to 0.91; p = 0.03; Figure 3).
Estimated blood loss
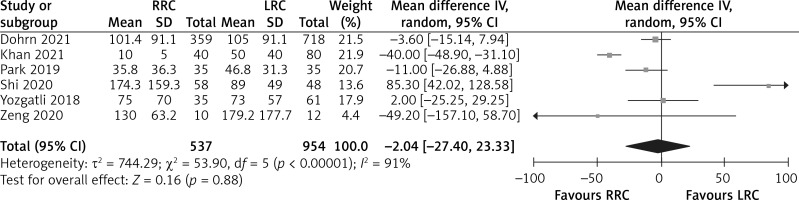
The estimated blood loss was reported in 6 studies. The results of the meta-analysis showed that there was no difference in estimated blood loss between the RRC group and the LRC group (MD = –2.04; 95% CI: –27.40 to 23.33; p = 0.88; Figure 4).
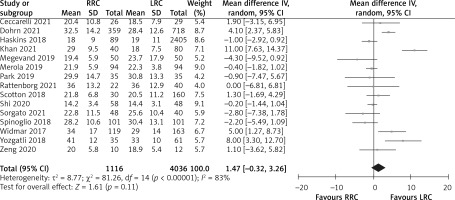
Retrieved lymphatic nodes
The number of retrieved lymphatic nodes was reported in 15 studies. Meta-analysis showed that there was no difference in the number of retrieved lymphatic nodes between the RRC group and LRC group (MD = 1.47; 95% CI: –0.32 to 3.26; p = 0.11; Figure 5).
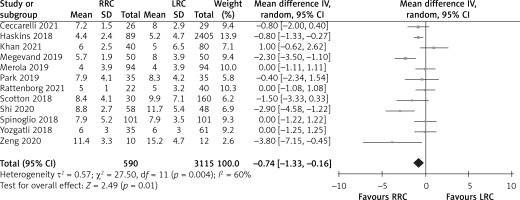
Length of hospital stay
The length of hospital stay was reported in 12 studies. The results of the meta-analysis showed that the LRC group had a significantly longer length of hospital stay than the RRC group (MD = –0.74; 95% CI: –1.33 to –0.16; p = 0.01; Figure 6).
Overall postsurgical complications
Overall postsurgical complications were reported in 14 studies. Complications developed in 245 (23.8%) patients in the RRC group compared with 381 (23.3%) patients in the LRC group. The results of the meta-analysis showed that the overall postsurgical complications were similar between the RRC and LRC groups (OR = 1.01; 95% CI: 0.83 to 1.24; p = 0.89; Figure 7). In terms of ileus, anastomotic leakage, and wound infection, the meta-analysis showed that there was no difference between the 2 groups (OR = 0.91; 95% CI: 0.60 to 1.39; p = 0.66; OR = 0.95; 95% CI: 0.54 to 1.67; p = 0.85 and OR = 0.88; 95% CI: 0.57 to 1.37; p = 0.58; Figure 7).
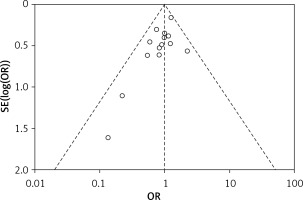
Publication bias
A funnel plot of overall postoperative complications was used to evaluate the presence of publication bias. The funnel plot was not asymmetric, indicating no evidence of publication bias (Figure 8).
Discussion
Laparoscopic surgery has been developed in the colorectal field for more than 30 years, and its safety, efficacy, and feasibility have been confirmed by several randomized controlled trials [27, 28]. At the same time, laparoscopic surgery has many advantages such as smaller incision, faster recovery, clearer field of vision, and thorough dissection, and it has become the first choice for most colorectal cancers. However, in clinical practice, laparoscopic surgery still faces limitations such as difficult operation in a small space, high requirements for team cooperation, long learning curve, and unstable images, which provides a new development space for the application of robotic systems in minimally invasive surgery. The robotic surgery system can overcome some of the limitations of laparoscopic surgery. The characteristics of the robotic surgery system make the operation more precise and delicate, especially in the separation and ligation of small blood vessels and the anastomosis of small lumen. Furthermore, the visual direction of robotic surgery is bottom-up, rather than top-down as in traditional laparotomy, which is more conducive to exposing the dorsal colon tissue. In addition, the learning curve of the robotic surgery system is shorter than that of the laparoscopic technique, the operation is easier to learn, and it can easily perform the difficult actions required in laparoscopic surgery. In recent years, the application of robots in colorectal cancer surgery has increased year by year [29], but its efficacy and safety remain controversial.
A total of 15 studies compared the efficacy and safety of RRC (1116 patients) and LRC (4036 patients), and they were all included in this meta-analysis. In terms of operation time, the RRC group was longer than the LRC group. The potential reasons for longer operation time include initial unfamiliarity with the robotic surgical system, poor trocar insertion experience, longer robot installation time, and difficulty in coordinating medical care [30]. De ‘Angelis [31] divided 30 cases of robotic surgery and 50 cases of laparoscopy surgery into 3 groups according to the operation sequence. Before the 2 groups of 10 cases the LRC group operation time was superior to the RRC group, but after 20 cases the RRC group was shorter. After a certain number of operations are trained, the advantages of the robotic surgery system’s accurate and flexible operation can be fully exerted, and the operation time can be significantly shortened. The shortening of time is also related to the fact that the surgical system can filter out the natural tremor of the human hand while presenting a magnified 3D field of view, which is more convenient for intraoperative tissue positioning and grasping [32]. As the operator becomes more proficient with the robotic system, the operation time can be further shortened, even better than for laparoscopic surgery [33]. The rate of conversion to laparotomy was significantly lower in the robotic surgery group than in the laparoscopic group. The robotic surgical system with one lens arm and 3 robotic arms, which can rotate 540°, can perform fine operations in small spaces, improve the ability to deal with more serious adhesions, and reduce the rate of conversion to laparotomy.
There was no statistically significant difference between the robotic group and the laparoscopic group in terms of intraoperative blood loss. Although robotic surgery is thought to potentially reduce intraoperative bleeding, the chance of intraoperative mishandling is higher relative to the laparoscopic group due to its lack of force feedback. At the same time, with the popularization of complete mesocolon excision (CME) for right-side colon cancer, the intraoperative blood loss of laparoscopic surgery is reduced, so the advantages of robotic surgery in controlling intraoperative blood loss are not obvious [34]. Pathologic outcomes of retrieved lymphatic nodes could be used as landmarks of long-term oncologic outcomes. Because patients’ prognoses may be affected by the number of retrieved lymph nodes, retrieving enough lymph nodes is critical to the postoperative treatment of colorectal cancer. In our study, no difference was found regarding the total number of retrieved lymph nodes between the 2 groups, indicating that both methods can achieve the expected lymph node dissection effect.
The length of hospital stay in the RRC group was shorter than that in the LRC group, but there was no significant difference in postoperative complications between the 2 groups. It may be because the robotic surgery has a clearer visual field during the operation, and the robotic arm can complete fine operations in a small space, reducing the secondary injury during the operation, reducing the operation trauma and the postoperative pain, and speeding up the postoperative recovery. However, in terms of hospitalization costs, the price of robotic surgery systems is still relatively expensive, which is a major bottleneck in the current popularization and development [34].
However, in terms of long-term evaluation indicators such as 3-year survival rate, 5-year survival rate, and progression-free survival period, the included literature data are insufficient, and meta-analysis cannot be performed. Spinoglio et al. [23] reported that the 5-year overall survival time of the robotic group and the laparoscopic group were 77 months and 73 months, respectively, and the progression-free survival rates were 85% and 83%, with no significant difference between the 2 groups. Nevertheless, in patients with Union for International Cancer Control (UICC) stage III, the 5-year progression-free survival after robotic surgery was 81 months, which was significantly different from that of laparoscopic surgery. At the same time, the randomized controlled study by Park et al. [18] also confirmed that there was no significant difference in long-term survival between the robotic group and the laparoscopic group. However, the sample size of the 2 methods was small and their convincing power was low, and large-scale follow-up and analysis are still needed.
Several limitations exist in the present meta-analysis. First, almost all the articles included in this meta-analysis were retrospective, which limited the strength of the final conclusions. Therefore, the risk of important bias is relevant. Secondly, it is impossible to match the patients’ characteristics in most of the studies, and thus heterogeneity between the 2 groups. Thirdly, most of the literature presented an intergroup difference in terms of the technique used to perform the anastomosis, which could have significantly biased the comparison between the groups. Due to the differences in equipment resources and the technical level of the implementer among different research institutions, the meta-analysis results have some heterogeneity. For example, the high heterogeneity of operation time results may be due to several factors. On the one hand, the robot assistance system is an emerging technology, and each surgical group is affected by experience and surgical level, resulting in an inconsistent stage of learning curve, thus having an impact on the surgical results. On the other hand, different definitions of operative time in different surgical groups may also lead to heterogeneity. Finally, data on surgery-related costs are still based on a limited number of surgeries performed in different health systems from different countries, and it needs confirmation from further cost-benefit analysis.
Conclusions
This meta-analysis suggests that RRC has longer operation time but lower conversion rate and shorter length of hospital stay than LRC. High-quality, large-sample, multicentre, randomized controlled studies are needed to furtherly investigate the postoperative quality of life and long-term prognosis. It is believed that with the continuous improvement of robotic surgical systems and surgical technology, it is expected to become the standard of minimally invasive surgery for right-sided colon cancer.