INTRODUCTION
Drugs of abuse have many valuable properties and are used in therapy, e.g. morphine is a very good painkiller, amphetamine derivatives are used to normalize attention deficit disorder and to treat narcolepsy [1], and cocaine is used in nose and eye surgery [2]. However, these substances usurp the right to act in reward, motivation and memory circuits, and their abuse can lead to pathological neuroadaptive changes and, in susceptible individuals, to the development of addiction.
The hallmark of addictive substances is their rewarding effects. The rewarding effects of almost all addictive substances are associated with three ascending dopaminergic pathways: the nigrostriatal, the mesocortical, and the mesoaccumbal dopaminergic tracts [3, 4]. The mesocorticolimbic dopaminergic network may be critical not only for reward properties following acute administration of psychostimulant drugs but also for long-term neuro-adaptive changes in the brain responsible for behaviors characteristic of drug addiction, particularly cocaine addiction [5-8]. The rapid release of dopamine (DA) induced by psychostimulants is associated with the subjective sensation of the so-called “high” [9]. Rapid changes in DA levels activate dopamine D1 receptors with low affinity. Their activation is essential for the development of the rewarding effects of drugs [10] and the production of conditioning [11]. There also appears stimulation of high-affinity DA D2 receptors, but their stimulation is not sufficient to produce a rewarding effect [12, 13], and these receptors can even reduce reward perception [14, 15].
With the development of addiction, cognitive and motivational disturbances arise. Structures of dopaminergic limbic-cortical circuits, such as ventral tegmental area (VTA), medial prefrontal cortex (mPFC), nucleus accumbens (Acb), and amygdala (Amy) are involved in these processes [16]. They are responsible for impulsivity and reactivity to stimuli. Neuroadaptations involving these areas are thought to be crucial in the development of addiction [1, 17]. The VTA is responsible for the initiation of behavioral sensitization, reward-motivated behavior, or anhedonia in acute withdrawal; the mPFC, which is responsible for inhibitory control, is the site where the effects of drugs of abuse lead to altered executive function and impaired decision making; the Acb is responsible for reward action, maintenance of sensitization, and persistent drug-induced behavior; the Amy is associated with memory for the motivational impact of drug-associated stimuli [16, 18, 19]. Substances of abuse are associated with rewards, which have hedonic (“liking”) and motivational (“wanting”) values and drug intake is accompanied by the learning of associations between reward and drug-associated stimuli [20]. The value of natural rewards and the motivational aspects of intracranial self-stimulation-induced reward is controlled by serotonergic innervation of the limbic-corticostriatal circuit [21, 22].
5-HT plays an essential role in, among other things, associative learning, a process important for drug use behavior. It is exploited in a classic test for studying reward action, the conditioned place preference (CPP) test, in which animals learn to associate specific environment with a drug-related context. Drug-altered serotonin neurotransmission can contribute to vulnerability and altered neurobiological states that result in the transition from controlled use, through abuse, to addiction [23]. Serotonergic neuroadaptive changes induced by just the first dose of a drug can contribute to the development of addiction [24].
Two serotonergic pathways extending from their parent neurons from the raphe nuclei, the dorsal raphe nucleus (DRN) and median raphe nucleus (MNR), innervate cortical and subcortical structures [23]. These 5-HT neurons intensely innervate the forebrain (hippo-campus, hypothalamus, substantia nigra (SNc), medial mammillary nucleus, lateral septum, thalamus, AMY, and cortex) [25]. Dopaminergic brain regions such as the SNc and VTA, as well as their terminal fields (dorsal striatum, Acb, and frontal cortex; see above), are thus innervated by the 5-HT system [25, 26]. Hence the interest in the role of the serotonergic system in the emergence of behaviors associated with controlled drug use, the transition to compulsive use, and the development of addiction in which the dopaminergic system plays a key role.
It has been shown that increasing and decreasing 5-HT transmission can decrease and increase, respectively, the ability of drugs such as cocaine to elicit a dopaminergic response [27, 28]. Most psychostimulants with addictive potential have been shown to increase 5-HT activity in almost the entire brain. Psychostimulants bind to the serotonin transporter (SERT), which leads to the inhibition of 5-HT reuptake and increased extracellular 5-HT outflow [29, 30]. This has significant effects on the cellular activity of raphe 5-HT neurons [31, 32] and neuronal signaling in terminal regions of raphe 5-HT neurons, such as cortical areas [33-35]. Thus, cocaine exposure significantly alters the function of 5-HT neurons and 5-HT signaling in limbic-cortical-striatal circuits and is thought to affect 5-HT neurons’ control of reward signal processing. Based on electrophysiological studies in monkeys [36], 5-HT DRN neurons have been shown to transmit reward-related information to the forebrain, and in studies in rats they have been shown to influence reward-seeking behavior [37-39].
Cocaine has been shown to cause 5HT release from the Acb [40, 41], dorsal striatum [33], ventral pallidum [42], hippocampus [41, 43], thalamus [44], hypothalamus, VTA [34, 45], and DRN [34]. Cocaine also increases extracellular 5-HT levels in several areas of the new cortex, such as the PFC [35, 46], the occipital and temporal cortices [47], and the entorhinal and perirhinal cortices. Taken together, acute cocaine administration increases extracellular 5-HT levels in the brain. Repeated cocaine administration may lead to increased SERT activity in terminal fields and decreased tissue and extracellular 5-HT levels in these areas. Also, AMPH increases extracellular 5-HT levels in the Acb and striatum [48-51], in the frontal cortex (FC) [52], PFC [35, 52], and entorhinal and perirhinal cortex, in parallel with locomotor activation [53]. During local intra-DRN administration of AMPH [54], increased levels of extracellular 5-HT were observed in the DRN but not in the striatum [55]. Local application of AMPH by reverse dialysis to the infralimbic and anterior cingulate subregions of the PFC also increased extra-cellular 5-HT levels in these regions [56]. Overall, acute administration of AMPH causes a significant increase in extracellular 5-HT levels in the brain. There is no evidence for up-regulation of SERT and a change in basal 5-HT levels after chronic use.
Serotonin (5-HT) has been shown to be essential not only for maintaining synaptic plasticity across the lifespan [57] but also for hedonic tone, motivation and reinforcement, and learning and memory [58]. Learning and memory, but also sensory processing [52, 59], temporally coincide with increased 5-HT activity. This analogy points to a functional role of brain 5-HT activation in the emergence of addiction.
EFFECTS OF PHARMACOLOGICAL MANIPULATIONS OF 5-HT SYSTEM ACTIVITY ON THE EFFECTS OF PSYCHOSTIMULANTS
In order to study the serotonergic mechanisms involved in the neurochemical and behavioral effects of psychostimulants, preclinical studies use pharmacological manipulations involving the administration to rats, peripherally or to selected brain structures, of substances that increase or decrease levels of 5-HT.
Decreasing 5-hT levels
Reducing 5-HT transmission may increase the behavioral effects of cocaine by increasing self-administration and enhancing cocaine-induced locomotor activity, CPP, and a goal-directed conditioned approach [27, 60]. The most commonly used approaches to lower serotonin levels include: (i) reducing the substrate for its synthesis by administering a tryptophan-deficient amino acid mixture (acute tryptophan depletion) orally (rats, humans); (ii) decreasing its synthesis by administering an inhibitor of tryptophan hydroxylase, the rate-limiting enzyme in serotonin synthesis (rats); (iii), depletion of serotonin by administration of the neurotoxin 5.7-dihydroxytrypta-mine (5.7-DHT) (rats).
Studies that lowered 5-HT levels in rats using the tryptophan hydroxylase inhibitor para-chlorophenylalanine [61, 62] showed an increase in cocaine-induced hyperlocomotion. A similar effect was also observed after the lesion of serotonergic neurons using 5.7-DHT [63]. In contrast, after intracerebroventricular (ICV) administration of the neurotoxin 5.7-DHT, decreased levels of 5-HT were observed in the midbrain but not in the Acb or Amy. This was accompanied by an increased breakpoint [64]. Lesions of specific regions allowed researchers to determine their contribution to the effects of 5-HT on psychostimulant effects. Local decreases in 5-HT in the medial forebrain or Amy resulted in an increased breakpoint [65], indicating that 5-HT innervation in the forebrain is crucial for cocaine reward. Administration of 5.7-DHT to the mPFC attenuated the hyperlocomotor effects induced by cocaine, whereas lesions in the entorhinal and occipital cortices had no effect [66]. Both global damage of 5-HT neurons induced with 5.7-DHT, and that restricted to the forebrain or amygdala nuclei, increased responding for cocaine in a fixe and progressive ratio schedule in rats [65].
In rats, a tryptophan-deficient diet significantly increases locomotor activity, CPP, and dopaminergic responses to amphetamine and cocaine [67-69]. In humans, a diet devoid of tryptophan also increased cocaine-induced “craving” for the drug [69] but suppressed the “high” [70]. Using PET (11C) raclopride methods, it was shown that acute tryptophan depletion induced by the oral administration of a tryptophan-deficient amino acid mixture (acute tryptophan depletion) increased cocaine-induced drug seeking and striatal DA response to cocaine in cocaine-dependent humans [69, 70]. These authors believe that the dopaminergic effects of cocaine under conditions of acute tryptophan deficiency show some neuroanatomical specificity. The ventral striatum receives dense input signals from the Amy, hippocampus, and limbic cortex. They are thought to be responsible for maintaining motivation to act in response to salient stimuli, such as drug reward [1, 71, 72]. In contrast, the dorsal striatum receives more stimuli from association and sensorimotor areas of the cortex and less from limbic areas. Dopamine within the dorsal striatum is implicated in stimulus-response habit learning [73]. This may suggest that limbic system-mediated incentive motivational processes extend gradually from the ventromedial striatum to more dorsal area [74]. At low concentrations of 5-HT, drug-induced dopaminergic responses are increased within the dorsal striatum, resulting in increased motivation for drug reward and susceptibility to compulsive drug-seeking (see Figure I).
Figure I
Under condition of low concentration of 5-HT (tryptophan deficient diet), cocaine-induced dopaminergic responses are increased within the dorsal striatum resulting in increased susceptibility to compulsive drug seeking. Incentive motivational processes extend gradually from ventromedial striatum to more dorsal aspects
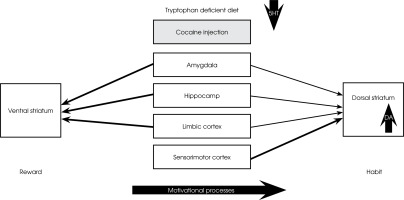
In humans, decreased 5-HT levels decreased resting limbic DA levels and increased the cocaine-induced DA response in the striatum and the desire to take the drug [69]. Most of the available studies done in rodents suggest that decreased tissue levels of 5-HT exacerbate most of the behaviors associated with cocaine use, such as hyperloco-motion, CPP, and self-administration relapse. This suggests that 5-HT suppresses cocaine use behaviors [23, 47]. However, it is essential to note that a systemic reduction in 5-HT does not necessarily equate to reducing the dynamic extracellular 5-HT response. In addition, the 5-HT synapse becomes hypersensitive to dynamic increases in 5-HT and responds with greater signal throughput to acute cocaine administration. While basal 5-HT tissue levels are reduced, the 5-HT synapse is rendered supersensitive towards a dynamic 5-HT increase and responds now with enhanced signal throughput to acute cocaine administration. Besides this, lowering basal 5-HT levels can increase the expression and sensitivity of 5-HT receptors [24, 75].
Given that repeated cocaine administration coincides with a decrease in tissue levels of 5-HT but exacerbates cocaine use behavior compared to that observed after acute cocaine administration [24], it is speculated that the acute increase in extracellular 5-HT after cocaine is not necessarily required to induce cocaine-use behavior but is necessary to cause the up-regulation of SERT in terminal areas and down-regulation at a somatodendritic level. This ultimately leads to a down-regulation of basal 5-HT levels, which involves hyper-sensitization of the 5-HT receptors. It has been suggested that this scenario will promote the development and maintenance of various cocaine use behaviors [24].
Increasing 5-hT activity
In contrast, increasing 5-HT neurotransmission has the opposite effect, decreasing cocaine-induced dopaminergic responses and behaviors [69, 76, 77]. Increasing 5-HT activity can be achieved by using a 5-HT precursor such as L-tryptophan and, even better, 5-hydroxy-L-tryptophan, using serotonin reuptake inhibitors (SSRIs) or 5-HT releasing agents such as fenfluramine.
Pradhan and colleagues [78] found that 5-hydroxy- L-tryptophan, a direct precursor of 5-HT, attenuates the locomotor response of cocaine in rats. Similar effects were obtained after administration of L-tryptophan [79]. Pretreatment with L-tryptophan decreased the breakpoint in a progressive ratio schedule of i.v. cocaine self- administration in rats [80].
Also, administration of fenfluramine, which releases 5-HT [81], or the selective 5-HT reuptake inhibitor (SSRI) fluoxetine [77, 82, 83] suppressed the reinforcing and motivational properties of cocaine. However, in other studies fluoxetine and fluvoxamine increased cocaine-induced locomotor activation [62, 84], although the administration of citalopram and sertraline was without effect [85]. The non-serotonergic mechanisms of action of fluoxetine appear to be responsible for the cocaine-enhancing effects of fluoxetine, as this effect was also observed in animals with 5.7-DHT-induced 5HT depletion [85].
In a study conducted in rhesus monkeys, PAL-434 (a 5-HT releasing substance) selectively reduced cocaine self-administration if the food was the choice [86]. The inhibitory effects of 5-HT on the motivational/ reinforcing effects of cocaine were also studied in SERT knock out (KO) in mice and rats. However, increased cocaine-induced CPP was observed in SERT KO mice and rats [87-89]. Compared to wild-type rats, the SERT-KO ones showed increased cocaine self-administration under short access conditions [87], and SERT-KO rats even escalated cocaine self-administration under long access conditions. Due to the lack of SERT and the associated lack of 5-HT reuptake, these rats have high extracellular 5-HT levels [90, 91]. However, tissue levels of 5-HT and 5-HIAA are lower in SERT-KO rats in the hippocampus, dorsal striatum, cerebral cortex and Amy, compared to wild-type rats [90]. Also, the electrically-evoked 5-HT release was lower in SERT-KO rats in the hippocampus, dorsal striatum, cortex and Amy [90]. Furthermore, such genetically altered rats showed more intense cocaine- seeking during extinction sessions.
THE ROLE OF 5-HT RECEPTORS IN THE EFFECTS OF PSYCHOSTIMULANTS
The high density of 5-HT receptors in structures of the mesocorticolimbic system, which is central to the development of addiction, suggests that 5-HT may play an essential modulating role [92, 93]. The role of the serotonergic system in the effects of addictive substances is complex. The action of 5-HT at the synapse is controlled by SERT; 5-HT neurotransmission is mediated by at least 14 different 5-HT receptor subtypes. These include 5-HT autoreceptors located at 5-HT nerve terminals that regulate 5-HT release [94, 95] as well as heteroreceptors located on dopaminergic terminals, dopaminergic, glutamatergic, GABAergic or cholinergic neurons that regulate the release of these neurotransmitters [96-98]. The 14 genetically encoded 5-HT receptor subtypes form seven families (5-HT1R – 5-HT7R) depending on their structural (mRNA and amino acid sequence homology) and functional (signal transduction mechanisms, pharmacological properties) properties (for review see [99, 100]). A further example of a complication is that 5-HT2CR can form 32 mRNA isoforms that can encode up to 24 different receptor protein isoforms in humans [101] and rats [102]. The 5-HT3R subtype is a ligand-gated ion channel, and the remaining 5-HTRs belong to a superfamily of receptors with seven transmembrane bands, more commonly referred to as G protein-coupled receptors (GPCRs) [103].
5-HT1B receptors
5-HT1B receptors appear to play an important role in mediating the effects induced by psychostimulants. Results regarding the effects of serotonin-5-HT1B receptor manipulation on the various effects of cocaine appear divergent. 5-HT1BR antagonists inhibit psychostimulant-induced locomotor activation [104-106] but 5-HT1BR KO mice show increased locomotor activation compared to wild-type mice [106]. Perhaps the reason for these differences is the different effects produced by acute pharmacological blockade and long-term genetic knockout of 5-HT1B receptors [105]. Interestingly, as mentioned above, the effects observed in SERT-KO animals were also opposite to those expected [24]. 5-HT1B receptor antagonists inhibit cocaine-seeking or cue-induced behaviors. Importantly, this effect of 5-HT1B receptor antagonists appears to be specific, as it does not affect other responses to cocaine (sensitization, CPP, self-administration maintenance), or food intake behaviors in rats [105]. In contrast, rats with an overexpression of 5-HT receptors showed increased cocaine reward for cocaine-induced CPP [107]. Acute pharmacological blockade of 5-HT1B receptors did not affect cocaine self-administration in mice [104] and rats [108, 109], or amphetamine in rats [110]. It has been shown [105, 111] that the 5-HT receptor antagonist GR 127935, administered at 3 mg/kg, did not affect cue- induced cocaine relapse. However, the 5-HT receptor anta gonist administered at a higher dose (10 mg/kg), and another antagonist, SB 216641 (7.5 mg/kg), strongly reduced psychostimulant-seeking behavior under cocaine provocation [112]. There are suggestions that these receptors undergo, bidirectional, changes dependent on abstinence status[113-115]. This is indicated by studies showing that 5-HT1B receptor agonists administered to the Acb increase cocaine reinforcement during self- administration and decrease cocaine-seeking and reoccurrence of cocaine-seeking behaviors when administered during abstinence [114, 115].
5-HT2 receptors
The 5-HT2R family consists of 5-HT2AR, 5-HT2BR and 5-HT2CR, which share sequence homology, similar pharmacological properties, and signaling pathways [99, 100]. The ultimate level of 5-HT2R functionality depends on many factors, including the availability of an active receptor pool and effective coupling and activation of signaling components.
These 5-HT2AR and 5-HT2CR receptors control the neurochemical and behavioral effects of psychostimulants, particularly the effects of cocaine. Preclinical studies indicate that 5-HT2AR antagonists and/or 5-HT2CR agonists can effectively reduce drug craving and/or relapse as well as increase abstinence, while 5-HT2CR agonists can also effectively reduce cocaine intake in active cocaine users [116]. 5-HT2AR and 5-HT2CR play important stimulatory and inhibitory roles, respectively, in modulating cocaine and other psychostimulant-induced DA output. Systemic administration of 5-HT2CR antagonists potentiated cocaine-induced DA release in the Acb [117] and the selective 5-HT2AR antagonist SR 46349B decreased cocaine [118] or amphetamine- induced DA release in it [119]. Consequently, these receptors can be expected to modulate behavioral effects induced by psychostimulants, in which dopaminergic mechanisms play a major role. The study of the neurobiological mechanisms of action of drugs often uses loco-motor activation related to their ability to release DA in the Acb (for a review, see [120]). Acute systemic injections of selective 5-HT2AR antagonists and 5-HT2CR agonists at doses that did not themselves affect behavior have been shown to block the hyperlocomotor effects of cocaine. In contrast, the administration of 5-HT2AR agonists and 5-HT2AR antagonists enhanced them [121-124]. 5-HT2AR and 5-HT2CR ligands similarly affected the effects of amphetamine [125, 126] and its derivatives (MDMA; ecstasy) [110, 127, 128], even though these substances have different mechanisms of action. Furthermore, the effects of 5-HT2 receptor manipulation have also been more similar for psychoactive substances other than psychostimulants, such as morphine, nicotine, and phencyclidine [116]. The effects of 5-HT2R agonists and antagonists on performance in other behavioral tests (drug discrimination, self-administration, cocaine reinstatement, cue reinstatement, CPP) were summarized in a review paper by Bubar and Cunningham [116]. The results indicate that 5-HT2AR receptors play an excitatory role and 5-HT2CR receptors an inhibitory role in the control of behavior induced by stimulant administration.
To identify the pathways and structures responsible for these effects, ligands specific for 5-HT2AR and 5-HT2CR were injected into different brain regions, starting at the DA mesocorticoaccumbens pathway’s DA nodes: the VTA, Acb or PFC [116]. Ligands were administered at doses that do not alter baseline behavior [129-131] but do alter cocaine-induced behavior when microinjected into the nuclei of the mesocorticoaccumbens pathway immediately before systemic cocaine administration.
Microinjection of the selective 5-HT2AR antagonist M100907 into the VTA inhibited the locomotor stimulant and discriminative stimulus effects of cocaine [129], as well as neuronal activation in the nucleus accumbens shell (AcbSh) assessed by Fos protein expression induced by systemic cocaine administration [132]. Intra-VTA infusion of another 5-HT2AR antagonist, SR 46349B, also blocked amphetamine-induced hyperactivity and amphetamine-induced DA release in the Acb [119]. In contrast, microinjection into the Acb of the selective 5-HT2AR antagonist M100907 did not affect cocaine-induced hyperactivity [129]. These results suggest that the 5-HT2AR in VTA plays a pivotal role in controlling psychostimulant-induced hyperactivity, possibly through its effects on psychostimulant-induced efflux DA, but the role of the 5-HT2AR in Acb and PFC is not yet clear.
The 5-HT2CR antagonist RS102221, administered into the VTA, did not alter cocaine-induced hyper-activity [130, 133], whereas the infusion of a 5-HT2CR agonist blocked it [85]. In contrast to the inhibitory effects of 5-HT2CR agonists in the VTA, an intra-AcbSh microinjection of the 5-HT2CR antagonist RS 102221 attenuated, whereas infusion of the 5-HT2CR agonist MK 212 or RO 60-0175 potentiated, the hyperlocomotor effects of cocaine in a dose-dependent manner [130, 133]. In contrast to that observed after systemic administration [122, 123], intra-mPFC microinjections of the 5-HT2CR agonist MK 212 or the antagonist RS 102221 decreased or increased the hyperlocomotor effects of cocaine, respectively [131]. The effects of 5-HT2CR agonists, when administered systemically and to the PFC, are similar and exert inhibitory control over the hypermotor effects of cocaine. The use of microinjections of 5-HT2CR ligands into individual structures indicate that 5-HT2CR in the Acb and PFC have to oppose stimulatory and inhibitory effects on the hyperlocomotor effects of cocaine, respectively. The effect induced by administration of 5-HT2CR ligands depends on the contribution and interplay of the different 5-HT2CR populations located in the VTA, Acb, and PFC, with the 5-HT2CR population in the PFC appearing to have a dominant influence.
In summary, the results of microinjection studies indicate that while 5-HT2AR and 5-HT2CR in the mesocorticoaccumbens nuclei do not play an active tonic role in behavioral control, separate populations of 5-HT2AR and 5-HT2CR within the PFC, Acb, and VTA differentially affect the output of the mesocorticoaccumbens DA pathway. These studies indicate that the VTA is one of the sites in the brain in which 5-HT2AR exerts an excitatory effect on cocaine-induced behavior, whereas the PFC is a specific site in the brain in which 5-HT2CR exerts an inhibitory effect on cocaine-induced behavioral responses [131, 134]. However, further studies evaluating the involvement of 5-HT2ARs and 5-HT2CRs located in brain areas outside the mesocorticoaccumbens circuit are necessary to understand the specific sites of action of these areas’ receptors throughout the brain.
5-HT2BRs also appear to play an important role in regulating both basal and “stimulated” DA activity. In particular, data available in the literature show that 5-HT2BRs are able to regulate DA release and transmission, as well as DA-dependent behavior, through complex polysynaptic processes [135].
Locally differential effects of 5-HT2BR antagonists on dopaminergic activity in the mPFC and Acb have been demonstrated. This is likely due to a functional interaction of 5-HT2BR with 5-HT1ARs involving cortical-sub-cortical pathways. It has been observed that the blockade of 5-HT2BR results in an increased cortical outflow of 5-HT, which stimulates 5-HT1ARs located on GABA-ergic interneurons in the mPFC [136] and consequently stimulates pyramidal glutamatergic neurons [137], leading to opposing changes in DA outflow from the mPFC and Acb through direct or indirect interactions with DA VTA neurons [58]. This suggests a functional role for a specific 5-HT2BR population in the regulatory control of DA neuronal activity. Although the cellular mechanisms involved in this interaction have not yet been established, it is noteworthy that 5-HT2BRs exert independent control over the activity of three ascending DA pathways through specific tonic excitatory and inhibitory control of DA efflux from the Acb and mPFC, and that there is no effect in the striatum.
CONCLUSIONS
Research over the years has led to significant advances in the understanding of serotonergic mechanisms involved in the effects of psychostimulants. So far, it has been established that pharmacologically decreasing or increasing the activity of the 5-HT system leads to an increase or decrease in the addictive effects of psychostimulants. Studies using 5-HT receptor ligands have provided much interesting information. There are suggestions that 5-HT1B receptors undergo bidirectional, abstinence- dependent changes. While 5-HT1B receptor agonists administered to Acb during self-administration increase cocaine reinforcement, they decrease cocaine-seeking and the reoccurrence of cocaine-seeking behavior when administered during abstinence.
In contrast, separate populations of 5-HT2AR and 5-HT2CR within the PFC, Acb, and VTA differentially affect the output of the mesocorticoaccumbens DA pathway. These studies indicate that the VTA is a brain site upon which 5-HT2AR exerts an excitatory effect on cocaine- induced behavior, whereas the PFC is a specific brain site upon which 5-HT2CR exerts an inhibitory effect on cocaine-induced behavioral responses. In contrast, 5-HT2BR exert independent control over the three ascending DA pathways’ activity through specific tonic excitatory and inhibitory control of DA efflux from the Acb and mPFC, with no effect in the striatum. These findings indicate that by manipulating the activity of the serotonergic system we can increase and, more importantly from a therapeutic point of view, decrease the effects of psychostimulants. However, many substances with therapeutic potential, such as 5-HT2BR ligands, show strong side effects. Despite considerable progress, the current state of knowledge does not translate into efficacy in the treatment of addiction, and we still do not have an effective pharmacotherapy. This may be because we study selected elements of a very complex system and do not cover the entire cascade of events caused by acting on a selected point of this complex machinery.







