INTRODUCTION
Montelukast has been approved for 27 years for the treatment of asthma, including asthma with concomitant allergic rhinitis. In many countries outside Poland, it is also indicated for the management of isolated allergic rhinitis. In Poland, it is approved as an oral formulation for children from 6 months of age, though not currently available. Chewable tablets are available for patients from 2 years of age.
MECHANISM OF ACTION
The drug blocks the cysteinyl leukotriene receptor Cys-LTR1, which is responsible for the pro-inflammatory effects of cysteinyl leukotrienes, the primary lipid mediators of inflammation in asthma [1–4]. Montelukast inhibits one of the two key inflammatory pathways mediated by 5-lipoxygenase. The pathway is responsible for:
increased vascular permeability,
increased production of bronchial mucus,
bronchospasm,
eosinophilic inflammatory infiltrate formation,
bronchial remodelling.
Importantly, the aforementioned inflammatory pathway is not suppressed by inhaled or systemic glucocorticosteroids. Furthermore, biological drugs exert only a selective, drug-dependent, and incomplete effect on this pathway [5].
It should be emphasised that patients with asthma, as well as those with both asthma and allergic rhinitis, produce higher levels of cysteinyl leukotrienes compared to healthy individuals [6].
BENEFITS OF ANTILEUKOTRIENE THERAPY IN ASTHMA MANAGEMENT
Inhaled corticosteroids (ICS) used in asthma treatment provide a highly effective and broad anti-inflammatory action. However, numerous in vivo and in vitro studies indicate that they have minimal impact on cysteinyl leukotriene production in both the lower and upper airways. Consequently, relying on a single anti-inflammatory drug to manage bronchial asthma is often ineffective. This results in approximately 40% of asthma patients lacking full control over their disease. In many patients asthma control is difficult to achieve, even when LABA/ICS or LAMA/LABA/ICS combination are utilized. It is important to emphasize that even with biological treatment, based on a general analysis of T2 and non-T2 asthma phenotypes, the cysteinyl leukotriene-dependent inflammatory pathway is not fully controlled.
Furthermore, antileukotrienes are taken in tablet form, which tends to be more acceptable to patients compared to inhaled medications, leading to higher levels of adherence. Adherence to and repeat prescriptions for montelukast are superior compared to the most commonly used inhaled combination therapies [7].
Inhaled medications, regardless of the inhaler type, carry a risk of administration errors. The inhaler type has minimal impact on preventing these mistakes. Oral dosage forms are less prone to medication errors [7–10].
Also, approximately 30–40% of asthma patients are overweight or obese, and the mechanics of breathing are often impaired in this group. As a result, the effectiveness of inhaled drugs in these patients is reduced. Due to significant extrapulmonary restriction, inhaled medications often fail to reach or only minimally reach the peripheral parts of the lungs. Montelukast is the only safe drug that effectively reaches the peripheral sections of the lungs in obese individuals [11].
A similar phenomenon is observed in pregnant women during the later stages of pregnancy, when the expanding uterus elevates the lower portions of the lungs. As a result, the deposition of inhaled drugs in the peripheral parts of the lungs is significantly reduced. In such cases, montelukast may offer an effective solution [12–16]. The drug is considered safe for use during pregnancy, irrespective of pregnancy stage [15].
Furthermore, montelukast is an approved and effective treatment for isolated allergic rhinitis in numerous countries [17]. In combination with antihistamines, montelukast demonstrates similar efficacy to intranasal corticosteroids (GCS) in the treatment of this condition [18–24]. In fact, more than 90% of asthma patients also have concomitant allergic rhinitis. The use of montelukast in individuals with asthma addresses their allergic rhinitis as well. This significantly enhances asthma control while improving patient adherence, and lowers treatment costs for both the patient and the payer.
Additionally, the high efficacy of montelukast in smokers should be emphasized, as GC treatment is approximately 50% less effective in these individuals compared to non-smokers [25]. Smoking does not impact the efficacy of montelukast in treating asthma. This is particularly important, as 35–50% of asthma patients are either active or passive smokers.
MONTELUKAST IN PAEDIATRIC PATIENTS
In many countries, the drug is approved for the treatment of asthma in children as young as 6 months of age. The youngest children receive it in granule form, older children in chewable tablets, and teenagers in coated tablets. This reflects the high safety profile of the drug. Notably, over 15 years ago, the Polish Society of Allergology, in its position paper, recommended montelukast as the first-line treatment for early childhood asthma. The drug is commonly used in children as an add-on to ICS or as monotherapy in patients who have used ICS at the lowest approved dose for at least 3 months without experiencing any symptoms during that period [23, 24, 26–30].
Leukotrienes are also released during exercise-induced bronchoconstriction. Therefore, exercise-induced asthma symptoms warrant a review of the patient’s current treatment. In many cases, adding antileukotriene medications to the treatment regimen can enhance disease control and help patients maintain an active lifestyle [31–37].
ADVERSE EFFECTS OF MONTELUKAST
Phase I, II, and III clinical trials have demonstrated that the drug is well-tolerated and does not cause significant adverse events. Typically, patients experienced headaches, skin rashes, or mild diarrhoea, while upper respiratory tract infections were uncommon. All symptoms were self-limiting and did not necessitate discontinuation of the medication [38–44]. During post-marketing surveillance of adverse drug reactions, no significant adverse effects were observed, except for a few anecdotal reports of nightmares. It is important to note that switching the administration of the drug from bedtime to morning resulted in the elimination of these adverse effects. Anecdotal data and results from small clinical trials were summarized in a paper by Schumock et al. [45]. Large-scale safety cohort studies were subsequently requested.
In 2019, a study was published indicating a nearly twofold increase in the incidence of CNS-related adverse events, primarily aggression, nightmares, and hallucinations. This study compared the incidence of these adverse effects between two groups, one of which took the drug and the other did not [46]. The obvious drawback of this study – the lack of assessment of the prior occurrence of the aforementioned behaviours and the level of daily asthma control – went unnoticed.
Another study, analysing data from adverse effect reports, found a higher incidence of sleep disturbances in young children using montelukast, aggression-related disorders in younger adolescents, and depression and suicidal thoughts in older adolescents [47]. It is important to note that patients’ medical data regarding concomitant diseases and neuropsychiatric disorders, both prior to and following montelukast withdrawal, were not analysed [47]. Similarly, a study involving a small group of approximately 100 patients, many of whom had pre-existing neuropsychiatric disorders before starting montelukast, yielded results that were challenging to interpret. This was further complicated by the authors’ apparent conflicts of interest and multiple post-publication corrections to the study [48–50].
In 2020, the U.S. FDA issued a black box warning for the drug, highlighting its potential to cause neuropsychiatric symptoms. This decision was prompted by reports of isolated mental health episodes observed during post-marketing surveillance in the U.S., as well as the low-evidence studies referenced earlier. However, it was not clarified whether individuals using the drug had previously been diagnosed with mental or psychiatric disorders. The analyses of concomitant diseases and the patients’ medical history are unknown. A widely reported case involved a young man who, in a state of agitation, ingested an excessive dose of montelukast and subsequently died by suicide. However, it is impossible to determine whether the drug contributed to the suicide or if both the drug use and the tragic outcome were related to the man’s underlying compulsive mental agitation.
Over 400 drugs in the U.S. now carry similar warnings, and this number has been growing significantly for several years [51]. The European Medicines Agency (EMA) has not yet mandated the inclusion of such a warning.
Since 2020, numerous studies have investigated the potential risk of neuropsychiatric symptoms associated with the use of montelukast in both children and adults. Extensive analyses of data from medical registries have found no link between montelukast and mental health disorders in children, adolescents, or adults.
The largest registry-based study to date included nearly 30,000 patients, tracking their health outcomes for 1 year before and 1 year after initiating treatment with the drug. The findings revealed that the incidence of neuropsychiatric events remained consistent during both periods. Therefore, montelukast cannot be directly linked to the occurrence of clinically significant neuropsychiatric events [52] (Figure 1).
Figure 1
Montelukast is not associated with an increased risk of neuropsychiatric adverse effects, including depression, suicidal ideation, aggressive behaviours, or other symptoms that may necessitate the use of medications prescribed by psychiatrists. A sequential analysis was performed before and after drug initiation, with a follow-up period of 52 + 52 weeks, according to [52]
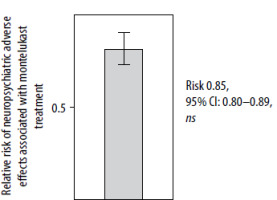
In conclusion, the alleged association between montelukast and psychiatric disorders, along with the inclusion of a warning on the drug leaflet, lacks a rational scientific basis. The data come from small clinical or observational studies, which are prone to considerable methodological flaws. They were disproven by a well-designed and well-conducted study involving nearly 30,000 patients [52].
It should be kept in mind that patients with chronic conditions like allergic rhinitis and asthma are more likely to experience behavioural disorders, depression, and suicidal thoughts. This should prompt physicians across all medical specialties, including paediatric allergists, pulmonologists, and internists, to identify such behaviours early and initiate appropriate diagnostic and therapeutic interventions.
CONCLUSIONS
Montelukast enhances asthma control in patients who do not achieve sufficient management with ICS or ICS/LABA. For children, it can be considered an alternative treatment to low-dose ICS treatment. It is particularly effective in smokers, individuals with obesity, pregnant women, and patients with exercise-induced bronchoconstriction.
It significantly improves the control of allergic rhinitis, and when combined with antihistamines, it is as effective as intranasal corticosteroids. It has few and mild adverse effects, does not lead to mental disorders, and does not disrupt sleep.







