Introduction
In a short time, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) completely changed the reality of not only inflammatory bowel disease (IBD) patients but also of the gastroenterologists treating them. Both of the diseases classified as IBD, i.e. ulcerative colitis (UC) and Crohn’s disease (CD), are chronic, incurable autoimmune diseases whose biology requires constant contact with healthcare providers [1, 2]. The lockdown measures, which necessarily involved isolation and limited access to healthcare services, significantly increased the level of stress in this group of patients [3, 4]. Coronavirus disease 2019 (COVID-19) is a disease that has reached every group of patients, despite the numerous restrictions and security measures. Although the data on the course of COVID-19 in patients with IBD, even in biologically treated patients, do not indicate a worsening of the course of the disease, it still raises many concerns [5–11]. To identify patients likely to develop severe COVID-19 and to facilitate medical decision-making, a multivariate model of predicting adverse outcomes was developed and validated [12]. The reality changed with the introduction of vaccines, which play a key role in the fight against the pandemic, but IBD patients have not been included in studies [13].
At the time of this study, 4 vaccine preparations against COVID-19 approved for use in the European Union by the European Medicines Agency were available in Poland: 2 mRNA vaccines (Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273) and 2 vaccines containing non-replicating viral vectors (AstraZeneca and COVID-19 Vaccine Janssen). As mRNA vaccines, Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 contain the RNA molecule of the target antigen, placed in a matrix made of lipid nanoparticles. After administration, the mRNA is incorporated into the host cells and translated into the SARS-CoV-2 spike protein, which strongly stimulates the humoral and cellular immune response. On the other hand, AstraZeneca and COVID-19 Vaccine Janssen, as vector preparations, contain modified genetic material that expresses the SARS-CoV-2 peak protein and stimulates the immune response as an antigen. The schedule of these vaccines is presented in Table I.
Table I
Characteristics of COVID-19 vaccination schedule and use
What have we already found out? Many different gastroenterology societies have recommended vaccination for IBD patients, because it is well tolerated by them [14–16]. A question that was raised not only by gastroenterologists but primarily by the patients themselves was whether they were effective, especially in connection with immunosuppressive and/or biological treatment. Patients who received high doses of glucocorticoids, immunosuppressants, and some biologics – such as infliximab – showed a weaker immunosuppressive response [17]. Also, the persistence of antibodies in this group of patients is not clearly defined, which is an important question in terms of booster doses [18]. Moreover, there are data on the seroprevalence of SARS-CoV-2 antibodies in IBD, but without the symptomatic course of COVID-19, which further complicates the differentiation of individual groups of patients [19].
Some IBD patients showed no interest in vaccinations due to their concerns about efficacy and safety [15, 20]. Others questioned their meaning due to the undergone COVID-19 [20]. A significant problem here was the lack of data on the persistence of SARS-CoV-2 antibodies in post-infection patients undergoing immunosuppression and/or biological treatment. This insufficient data led us to investigate the issue in our centre, which treats more IBD patients than other centres across Poland.
Aim
As employees of the largest IBD centre in Poland, we analysed whether the drugs used in the treatment of IBD patients may affect the concentration of SARS-CoV-2 antibodies, which was the primary endpoint of our study.
Material and methods
Study design
This was a prospective, single-centre assessment of SARS-CoV-2 neutralizing antibody (NAb) concentration in IBD patients following a full vaccination course. It used a DiaSorin Liaison SARS-CoV-2 S1/S2 IgG test – an indirect chemiluminescence immunoassay – for the quantitative determination of IgG S1 and IgG S2 antibodies specific to SARS-CoV-2 (negative values were < 15 AU/ml). Subsequent measurements took place at 2-month intervals for 6 months. Patients who reported prior confirmed COVID-19 infection status were not included in the study. The samples were collected in the period from 13 May 2021 to 17 December 2021.
Statistical analysis
Data analysis was carried out using R software, version 4.0.5. The variables are presented with descriptive statistics: count (n) and % frequency for nominal data and mean ± SD or median (Q1; Q3) for continuous data, as appropriate. The normality of distribution was validated with the Shapiro-Wilk test, skewness and kurtosis values, and a visual assessment of histograms. For the level of antibodies, all values below the lower detection limit were replaced with half of the corresponding detection limit value, while all values above the upper detection limit were replaced with the upper detection limit value multiplied by a factor of 1.2 [21]. Patients with and without vaccination were compared with a χ2 test or Fisher’s exact test for nominal variables and with a t-test for continuous variables. The relationship between the level of antibodies and the time from full vaccination was assessed using Spearman’s correlation coefficient.
Results
Of the 346 IBD patients enrolled in the study, 148 (42.8%) were female and 198 (57.2%) were male (Table II). The median age in the group was 39 years. The distribution of disease indicated more CD than UC: 206 (59.5%) cases vs. 140 (40.5%) cases, respectively. The vast majority of the group (271 (78.3%)) was vaccinated with Pfizer-BioNTech, while AstraZeneca was used in 27 (7.8%), Moderna in 25 (7.2%), and COVID-19 Vaccine Janssen in 22 cases (6.4%). Biologics were used in 248 (71.7%) patients, the most common being infliximab (122 (35.3%)). Other administered drugs were vedolizumab (66 (19.1%)), adalimumab (40 (11.6%)), ustekinumab (11 (3.2%)), and tofacitinib (9 (2.6%)). Mesalazine was used in the treatment of 210 (60.7%) patients, while more than half received immunosuppressive therapy: azathioprine (166 (48%)) or 6-mercaptopurine (31 (9.0%)). Glucocorticosteroids were used in 52 (15.0%) cases, but budesonide was used in only 23 (6.6%). Other drugs were used less frequently: methotrexate (9 (2.6%)), cyclosporin (2 (0.6%)), and mycophenolate mofetil (2 (0.6%)).
Table II
Characteristics of the group
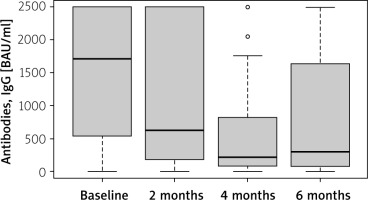
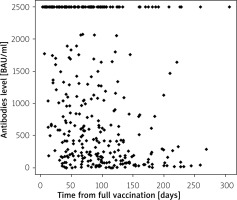
The antibody responses were measured at various time points after the full vaccination course was completed: every 2 months through the 6th month (Figures 1, 2). The median levels of antibodies over these time points are presented in Table III.
Table III
Antibody levels in the study group over time
A negative but moderate correlation between antibody level and time from full vaccination was confirmed in this group (rho = –0.34, p < 0.001). In patients vaccinated with Moderna or Pfizer-BioNTech, the same correlation was confirmed, though of different strength (rho = –0.50, p = 0.010 for Moderna and rho = –0.36, p < 0.001 for Pfizer-BioNTech). For AstraZeneca and COVID-19 Vaccine Janssen vaccination, no such correlation was confirmed. The patients with UC had a slightly stronger correlation between antibody level and time from vaccination (rho = –0.43, p < 0.001) than CD patients (rho = –0.30, p < 0.001). This correlation was confirmed in patients with and without biological therapy (rho = –0.36, p < 0.001, and rho = –0.40, p < 0.001, respectively). Looking at particular types of biological therapy, a correlation was confirmed for adalimumab (rho = –0.35, p = 0.025), infliximab (rho = –0.32, p < 0.001), and vedolizumab (rho = –0.50, p < 0.001). In cases where other drugs were administered long-term, a negative correlation between antibody level and time from full vaccination was confirmed for azathioprine (rho = –0.44, p < 0.001), budesonide (rho = –0.58, p = 0.004), mesalazine (rho = –0.35, p < 0.001), and systemic glucocorticosteroids (rho = –0.58, p < 0.001) (Table IV).
Table IV
Correlation between antibody levels in the study group and time since full vaccination
In patients taking both biologics and immunosuppressive drugs, a correlation was confirmed for the same types of biological therapy (adalimumab, infliximab, and vedolizumab). For patients on other long-term pharmaceutical therapies, a negative correlation between antibody level and time from full vaccination was confirmed for mesalazine (rho = –0.38, p < 0.001), budesonide (rho = –0.58, p = 0.024), systemic glucocorticosteroids (rho = –0.58, p < 0.001), and azathioprine (rho = –0.48, p < 0.001) (Table V).
Table V
Correlation between antibody levels and time since full vaccination in patients with biological therapy
In patients without biological therapy, a negative correlation between antibody level and time from full vaccination was confirmed for mesalazine (rho = –0.41, p < 0.001), systemic glucocorticosteroids (rho = –0.72, p = 0.009), and azathioprine (rho = –0.38, p = 0.006) (Table VI).
Table VI
Correlation between antibody levels and time since full vaccination in patients without biological therapy
Discussion
There is evidence of a diminished immune response to vaccination in IBD patients treated with glucocorticoids, immunosuppressants, and biological agents. In 2012, Fiorino et al. postulated that patients with IBD should be vaccinated against pneumococci before starting biological treatment, because they noticed that the use of infliximab or combination therapy (infliximab with azathioprine) significantly decreased the response to vaccination compared to mesalazine (57.6% and 62.5% vs. 88.6%, respectively; p < 0.05) [22]. Similar conclusions were reached in 2016 by deBruyn et al., who confirmed that only 45% to 80% of IBD patients treated with infliximab achieved serological protection against influenza as a result of vaccination. They emphasized how necessary vaccination is when introducing biological treatment and how important it is for doctors to inform patients with IBD about vaccination [23]. On the other hand, in their analysis published in 2018, Pratt et al. confirmed that people treated with infliximab or adalimumab had lower seroconversion rates than healthy people after receiving hepatitis B virus (HBV) vaccines. They also pointed to the need to vaccinate IBD patients against HBV before using immunomodulators and anti-tumour necrosis factor (anti-TNF) drugs – especially infliximab – and then to periodically screen them [24]. In the Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease, published in 2021 by the European Crohn’s and Colitis Organisation (ECCO), the use of dead vaccines is explicitly recommended in patients under immunosuppressive and/or biological treatment. In addition, the importance of vaccination against COVID-19 is emphasized [25]. In view of the above data, it is not surprising that anti-TNF drugs and azathioprine correlate with a reduction in NAb in the case of COVID-19 vaccination. However, it is surprising that the same correlation was found in the case of vedolizumab, which in a 2015 study by Wyant et al. was confirmed to not impair the immune response to parenteral antigens (HBV vaccine). However, a similar correlation was found in the case of the oral cholera vaccine, which is due to the selective mechanism of action in the gut [26].
Despite some research, the impact of biological treatment remains a topic of debate among gastroenterologists and IBD patients. In the United Kingdom, the multicentre CLARITY-IBD study, which enrolled 6935 patients, examined whether the drugs used in IBD (infliximab, vedolizumab, and/or accompanying immunomodulators, such as thiopurines or methotrexate) worsened the response to COVID-19 vaccination. Unlike our study (where the response was tested after 4 types of vaccination), the CLARITY-IBD study only looked at the response to Pfizer-BioNTech and AstraZeneca. The results clearly showed that patients treated with infliximab had lower NAb levels than those treated with vedolizumab. In our study, such an analogy was found in the case of anti-TNF drugs and vedolizumab, which is contradictory in terms of the local mechanism of action of this drug. The results of the CLARITY study confirm that the use of infliximab also resulted in a faster decrease in NAb [27, 28]. In our study, we also found lower levels of NAb in patients who used azathioprine, which is in contrast to the results of the above-mentioned study, where the recipients of the Pfizer-BioNTech vaccine had higher levels of antibodies regardless of the immunosuppressive drug used [29]. In the CLARITY study, no association was found between a lower percentage of NAb and drugs such as mesalazine, budesonide, or systemic corticosteroids.
Another international cohort study evaluating the response to vaccination with Pfizer-BioNTech or Moderna, the International Study of COVID-19 Antibody Response Under Sustained Immune Suppression in IBD (ICARUS-IBD), concluded that the treatment did not significantly affect the serological response. A total of 48 patients with IBD were enrolled in this study. After 2 doses of the vaccine, as many as 100% of the patients seroconverted regardless of the biological drug. Interestingly, in patients who had previously experienced COVID-19, higher levels of NAb were found after the first dose, which confirms the view that the infection itself acts as an immunization [30]. Another study that completely differed from ours is one by Kappelman et al., which included 317 patients with IBD. It found that none of the therapies used in IBD (biological treatment or immunosuppression) significantly influenced the response to COVID-19 mRNA vaccination, which was recorded in as many as 95% of the patients [31]. It was similar to a study by Shehab et al., in which no significant reduction in NAb was found after vaccination in 126 biologically treated patients who were present in 74% of infliximab-treated patients, 81% of adalimumab patients, almost 93% of vedolizumab, and all ustekinumab-treated patients after the second dose of Pfizer-BioNTech or AstraZeneca [32]. In our study, the results only confirmed the neutral effect of ustekinumab.
The greatest evidence that seroconversion rates after COVID-19 vaccination were similar despite different biological drugs among IBD patients remains the meta-analysis by Jena et al. published in 2022. They analysed 46 studies for seroconversion rates, positive neutralization, and breakthrough infections in IBD patients and compared them with control groups. Based on 31 studies with 9447 participants, the overall seroconversion rate after the full vaccination regimen was good (0.96; 95% confidence interval: 0.94–0.97) but slightly lower than in the control group (0.98; 95% confidence interval: 0.98–0.99). Positive neutralization test results were lower in IBD patients than in the control group. Some drugs, such as systemic glucocorticoids and the combination of TNF and immunomodulators, were associated with numerically (but not statistically) lower seroconversion rates as opposed to excellent seroconversion among the untreated patients or those receiving only anti-TNF drugs, vedolizumab, ustekinumab, or JAK inhibitors. A study on the persistence of antibody levels suggested that titres begin to decline 4 weeks after complete vaccination and may decline more rapidly with anti-TNF drugs, immunomodulators, or a combination of the two [33].
In the results of our study, statistical significance was found in the case of mesalazine (rho = –0.35, p < 0.001), which may be because it was used in as many as 60.7% of cases. Other studies have not confirmed its negative impact on NAb levels [33]. Therefore, it should be assumed that because almost half (48%) of the patients also took azathioprine, this relationship should be described as highly doubtful. This is mainly confirmed by the results of other studies, in which such a relationship was not confirmed [33]. A similar relationship seems to also exist in the case of budesonide (rho = –0.58, p = 0.004) and systemic glucocorticosteroids (rho = –0.58, p < 0.001). Due to the known local action of budesonide, it is not expected to affect the production of NAb, and there are no similar data on this effect of systemic corticosteroids [34].
The decline in antibodies level observed in our study was to be expected. It has been shown that messenger RNA vaccines stimulate durable immune memory; however, serum antibody responses steadily decline with a half-life of 56–66 days in the first 6 months after 2 doses, resulting in weak-to-undetectable NAb titres in many individuals [35–37]. In addition to the above, another limitation should be the examination of the level of antibodies, only in patients with IBD, but the lack of a control group was related to the then epidemiological situation. Hypothetically, among our patients, a subgroup treated with mesalazine alone (a non-immunosuppressive anti-inflammatory drug) might be used as a control group. We report, however, that in this subgroup (the number of patients taking mesalazine alone not given) a negative correlation between antibody level and time from full vaccination was also confirmed (rho = –0.38, p < 0.001) and was similar to other subgroups. The test we used in our study allows the detection of IgG antibodies against the S1/S2 (spike) antigens of the SARS-CoV-2 virus. These antibodies appear both after infection and after vaccination, so they are not very specific. To determine vaccine-specific antibody responses, we would currently use the anti-SARS-CoV-2 spike (S) immunoassay together with the nucleocapsid (N) immunoassay, with spike immunoassay positive but nucleocapsid immunoassay negative, which is consistent with vaccination without prior infection.
Conclusions
The results of this study clearly confirm the association between low titres of anti-SARS-CoV-2 antibodies after vaccination and certain drugs, i.e. systemic corticosteroids and biological agents such as infliximab and adalimumab. Choosing the right biological drug in IBD is still difficult and is based primarily on the doctor’s own experience and the course of the disease. However, rapid optimization of treatment to avoid exacerbations and the use of systemic corticosteroids remain crucial. In line with other studies of this type, there is still a need for gastroenterologists to inform patients about the need for booster doses, which is particularly associated with corticosteroid therapy and some biologics.