Introduction
For many years, the tools used to assess the patients’ quality of life (QoL) in various diseases have gained increasing interest. Their use has enabled physicians to observe how specific diseases impact patient’s social activities [1]. Dermatological conditions have been identified as some of the most detrimental to QoL, largely due to the visibility of skin lesions, which affects appearance and consequently impair emotional well-being and social interactions [2]. The development of the Dermatology Life Quality Index (DLQI) in 1994 marked a significant advancement in understanding the impact of skin diseases on QoL [3]. Currently, many national and international guidelines for managing dermatological diseases rely on the DLQI score [4]. Additionally, numerous other dermatology-specific health-related QoL (HRQOL) instruments, such as Skindex-29, the Dermatology Quality of Life Scales (DQOLS), and the Dermatology Specific Quality of Life (DSQL) have been developed to assess the impact of skin disorders on patients’ QoL [5–7]. While these instruments are important for comparing the quality of life across various diseases, specific scales, used to assess patients suffering from particular dermatoses or specific location, include more detailed questions that address the unique aspects and concerns of patients, and more accurately target the areas most impacted by the disease. Therefore, in 2002 Chen et al. [8] created SCALPDEX, a self-administered questionnaire, which was the first instrument to assess the impact of scalp dermatoses on the patients’ QoL.
Originally SCALPDEX was created in English.
Aim
The aim of this study was to translate and validate the Polish language version of the SCALPDEX questionnaire, to enable its use in clinical practice and research by Polish-speaking clinicians.
Material and methods
The Polish language version of the SCALPDEX questionnaire was translated and validated in line with international standards [9]. The permission to translate SCALPDEX into Polish was provided by the copyright holders. SCALPDEX was primarily developed to measure quality of life in patients with scalp disorders. It is a disease-specific questionnaire, containing 23 items, 9 of which were adapted from Skindex-29 and 14 were derived from interview session data. It was conceptualized into 3 major constructs that explain the way scalp dermatoses affect patients’ QoL: symptoms, emotions, and functioning. The possible answers are “never”, “rarely”, “sometimes”, “often”, and “all the time”. The answers are scored on a scale from 0 to 100 (0, 25, 50, 75, and 100). The final scores are calculated by the mean of the item scores relating to each construct. The higher the score, the more impaired the quality of life [8].
Translation and validation process
Initially, the original English version of the SCALPDEX questionnaire was translated into the Polish language by two independent translators, unfamiliar with the questionnaire. The resulting two initial versions were compared to create a more accurate third version. Afterwards, all three translated versions were compared in terms of inconsistencies by a bilingual expert in the field and the unified version was created. Subsequently, the back translation from the Polish version was performed. The reverse translation was discussed with the author of the original version of the questionnaire. As a result, a final Polish version of the SCALPDEX questionnaire was made.
In the next step, the validation process was performed. The questionnaire was distributed to a group of 60 patients from the Department of Dermatology, Venereology and Allergology in Wroclaw, Poland. Among these, men and women constituted 60% (36) and 40% (24), respectively. The age of participants ranged from 14 to 73 years (mean: 43.5 ±17.7 years). The majority of the respondents with scalp dermatoses were diagnosed as suffering from scalp psoriasis (n = 19), followed by seborrheic dermatitis (n = 11), alopecia areata (n = 7), squamous cell carcinoma located on the scalp (n = 6), dissecting cellulitis of the scalp (n = 6), cutaneous lupus erythematosus (n = 4), pemphigus vulgaris with scalp involvement (n = 4) and other skin disorders (n = 3) (Table 1). All patients were asked to fulfil the questionnaire twice within an interval of 7 days for reassessment to evaluate test-retest reliability. During this time, no interventions were conducted for the scalp disease.
Table 1
Study population characteristics
During the first completion, 30 patients were additionally asked to complete the Polish version of Dermatology Life Quality Index (DLQI), which served as a reference questionnaire to verify convergent validity [10]. DLQI was chosen as it was a pioneer questionnaire to assess QoL in dermatologic patients and is currently one of the most commonly used instrument among dermatologic patients.
Statistical analysis
Statistical analysis was performed to assess the reliability of the questionnaires. All the data analyses were carried out using the Statistica 13 (Dell, Inc., Tulsa, USA) software. The internal consistency was evaluated at the 1st administration of the questionnaire with Cronbach α coefficient. The questionnaire reproducibility (test-retest reliability) was assessed by comparison of the two responses of each patient with the use of intraclass correlation coefficient (ICC). It is believed that to prove that the questionnaire is internally consistent, the Cronbach α coefficient should be at least 0.7, while the values above 0.9 stand for very good internal consistency [11]. To indicate adequate reproducibility of the questionnaire, ICC, similarly to Cronbach α coefficient, should also be at least 0.7 [12].
The correlations between the answers from a single completion to each question and to the total score were verified with Spearman’s correlation test. The same test was used to measure the relationship between SCALPDEX and DLQI to check the convergent validity. Furthermore, responses to each question from the first and the second completion were compared with Wilcoxon test to search for important differences, with p-value < 0.05 considered as statistically significant.
Results
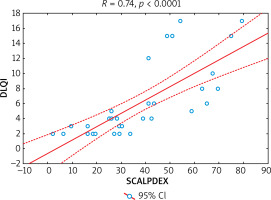
Assessment of internal consistency of the Polish language version of SCALPDEX demonstrated that all items of the questionnaire were strongly interrelated with each other. Cronbach α coefficient was very high and amounted to 0.94, which indicates very good internal consistency of the questionnaire. Exact the same value (0.94) was achieved for ICC indicating good reproducibility of SCALPDEX. The correlations between scores for each item and total scale score varied from 0.21 to 0.89 (mean: 0.63) (Table 2). In addition, no statistically significant differences were observed between the scores at the first and second administration of the questionnaire (with exception of Q5 and Q21) (Table 3). Moreover, there was also a strong positive correlation between SCALPDEX and DLQI scores (R = 0.74, p < 0.0001) proving the usefulness of SCALPDEX in reflecting the QoL in patients suffering from scalp dermatoses (Figure 1).
Table 2
Correlation of each item (Q) score with the total score of SCALPDEX
Table 3
Reproducibility of SCALPDEX results (of each item [Q] score and total score)
Discussion
Scalp dermatoses are frequently reported complaints in dermatological practice. They may emerge either as primary diseases of the scalp, as part of a generalized inflammatory skin disease, or as a manifestation of a systemic disease [13]. The scalp lesions may influence the patient’s appearance and raise fear, disgust or reluctance in other people and sometimes even they may be a reason of social rejection. This may cause emotional burden and result in a negative influence on the personal and social life of patients. The importance of proper QoL assessment in patients suffering from various scalp dermatoses should be therefore clearly emphasized [1, 14]. So far, many different dermatology-specific and disease-specific QoL instruments have been created. They are available as self-report questionnaires and used to assess the impact of the disease on QoL [4].
SCALPDEX is a group disease-specific tool to measure QoL in patients with scalp disorders [8]. In this study, we described creation and validation of the Polish language version of the SCALPDEX questionnaire. The analysis of internal consistency was accomplished on the basis of the results gained after a single completion of the questionnaire. Statistically significant, positive correlation between the results obtained for each question separately and for the total SCALPDEX score was established. The results regarding internal consistency were very good and the value of Cronbach α (0.94) was even higher than in the original version (0.62–0.80) [8]. Our results, however, are similar to those of Sampogna et al. [15], who proved a very good internal consistency of the instrument with Cronbach α coefficient value of 0.938. To the best of our knowledge, only the Italian version of the SCALPDEX questionnaire developed by Sampogna et al. [15] has been validated till now. Analysis of the reliability of the questionnaire was also performed, based on the responses of 2 administrations of the questionnaire within an interval of 7 days. A very good reproducibility was achieved with the value of ICC of 0.94, which was almost the same as in the original version (ICC = 0.90–0.97) [8]. Furthermore, besides only two questions (5 and 21), no statistically significant differences were observed between the total score and the score for individual questions obtained after completing the questionnaire twice. The evaluation of convergent validity showed a strong positive correlation between SCALPDEX and DLQI scores.
Conclusions
The Polish version of SCALPDEX has been demonstrated to be a reliable and valid instrument for evaluating QoL in patients with scalp disorders. Its high internal consistency, reproducibility, and strong correlation with DLQI make it a valuable tool for clinical practice and research in dermatology within the Polish-speaking individuals. This validation ensures a more accurate and appropriate assessment of the impact of scalp dermatoses, facilitating better patient management and care.