Summary
The current study constitutes one of the infrequent reports regarding the clinical relevance of ST-segment re-elevation (reSTE) following primary percutaneous coronary intervention (PCI) in patients with a spontaneously recanalized infarct-related artery (IRA). We found that reSTE was independently associated with both lack of improvement of global and infarct-related contractility as well as increase of left ventricular (LV) volumes in 6-month observation. Moreover, the procedure accompanied by reSTE was related to worse LV function and structure recovery as compared with patients with subsequent ST-segment resolution following PCI. Our findings provide evidence that myocardial injury reflected by reSTE is associated with the loss of beneficial effects of spontaneous IRA reperfusion on subsequent LV remodeling. Further studies on therapies to prevent reSTE following primary PCI are necessary.
Introduction
Spontaneous reperfusion characterized by a patent epicardial infarct-related artery (IRA) on initial angiography improves the prognosis in patients with myocardial infarction (MI). This phenomenon affects up to 30% of patients with ST-segment elevation myocardial infarction (STEMI) [1]. Based on the results of recent EUROMAX [2] and HORIZONS-AMI [3] trials, baseline thrombolysis in myocardial infarction (TIMI) 2 or 3 flow derived from spontaneous reperfusion was associated in contemporary practice with a higher rate of final TIMI 3 flow grade, decreased frequency of adverse cardiovascular events and reduced short- and long-term mortality. Similar beneficial effects were found with pharmacological reperfusion prior to percutaneous coronary intervention (PCI) [4]. According to the current guidelines [5] in patients with spontaneously normalized ST-segment elevation, angiography within 24 h is recommended. Moreover, recent studies showed that deferred PCI in spontaneously reperfused STEMI patients was associated with similar infarct size [6] and clinical outcomes [1, 6] as compared with immediate PCI.
The fourth universal definition of MI focused on the issue of myocardial damage associated with revascularization procedures. The procedure-related myocardial injury and MI associated with PCI (type 4a MI) were separated depending on the level of high-sensitivity cardiac troponin and the presence of new ischemic changes in ECG, cardiac imaging or angiographic findings of flow-limiting complications of PCI [7]. Previous studies reported that the incidence of peri-procedural MI varies from 5 to 30% and is associated with an increased risk of death (adjusted hazard ratio (HR) = 1.20, 95% CI: 1.04–1.39) [8]. When PCI is performed in a prothrombotic milieu in MI patients, it is extremely difficult to estimate the magnitude of myocardial injury associated with the procedure, while the extent of infarct-related necrosis is many times greater.
It was found that ST-segment re-elevation (reSTE) following primary PCI observed in one fifth of patients with anterior STEMI with an occluded IRA on initial contrast injection was not associated with increased infarct size, left ventricular (LV) function or clinical outcomes at 1 year [9, 10]. In contrast, lack of ST-segment resolution or reSTE within 24 h following PCI was associated with increased enzymatic injury and reduced LV function [11]. Moreover, in STEMI patients, microvascular obstruction and intramyocardial hemorrhage were more frequently observed in contrast-enhanced cardiac magnetic resonance of patients with reSTE during primary PCI [12]. Previous results also suggested that myocardial expansion of the infarcted portion [13] and pericarditis following MI may contribute to reSTE, but most of the available studies concerned patients with an occluded IRA.
Aim
We hypothesized that reSTE following primary PCI in a patent IRA might be associated with the loss of beneficial effects of spontaneous reperfusion. We sought to investigate the significance of reSTE during PCI in STEMI patients with a spontaneously recanalized IRA on LV function recovery and remodeling.
Material and methods
Of 969 consecutive STEMI patients we studied 155 (16%) patients with a patent IRA treated in one center with primary PCI. The inclusion criteria were chest pain onset within 12 h with concomitant ST-segment elevation of ≥ 1 mm in at least 2 contiguous leads or ≥ 2 mm in at least 2 contiguous precordial leads in qualifying ECG done during the first medical contact as well as initial TIMI 2 or 3 flow in the IRA on initial contrast injection during angiography. The exclusion criteria were cardiogenic shock on admission, previous coronary artery bypass surgery, history of malignancy, venous thromboembolism, liver injury (alanine aminotransferase > 1.5 ULN), serum creatinine > 177 µmol/l and current oral anticoagulation.
At first medical contact all patients received 300 mg of aspirin; 114 (73.5%) of them were loaded with 600 mg of clopidogrel and 88 (56.8%) received an intravenous bolus of 5000 IU of unfractionated heparin (UFH). On admission, hemoglobin, red blood cell count, white blood cell count, platelet count, glucose, creatinine, and high-sensitivity C-reactive protein were determined by routine laboratory techniques. Immediately before PCI, patients received a weight-adjusted bolus of UFH to achieve activated clotting time of 200–250 s. During primary PCI, 12 patients received abciximab as a bailout procedure. The Export Aspiration Catheter (Medtronic Inc., Minneapolis, Minnesota, USA) was used in 35 (22.6%) patients.
The study protocol complying with the Declaration of Helsinki was approved by the Ethics Committee of the Jagiellonian University. All subjects gave informed consent for their participation in the study.
ECG
Standard 12-lead ECGs (25 mm/s) obtained at the first medical contact (qualifying ECG (Q)), before PCI (B) and immediately after the procedure (A) were analyzed. The sum of ST-segment elevations in all leads (ΣST) was measured at the J point by a single investigator blinded to the clinical and angiographic findings. If the sum of STE following PCI was higher by at least 1 mm as compared with pre-PCI recordings, this patient was classified as re-elevated. Resolution of ST-segment elevation between qualifying ECG and ECG performed before (STRB) and after (STRA) primary PCI was calculated according to the formulas: STRB = 100%*(ΣSTQ – ΣSTB)/ΣSTQ and STRA = 100% × (ΣSTQ – ΣSTA)/ΣSTQ.
Angiography analysis
Angiogram analysis was performed off-line for the determination of the IRA, epicardial blood flow, thrombus burden and distal embolization, based on visual inspection. Epicardial blood flow was evaluated by means of the TIMI flow scale [14], myocardial perfusion was scored according to the TIMI myocardial perfusion grades (TMPG) [15] and thrombus burden was assessed according to the TIMI thrombus grades [16], all before and after primary PCI.
In addition, angiograms were analyzed for the presence of collateral flow to the IRA or the presence of flow-limiting lesions in non-IRA arteries. A detailed evaluation was performed in two contralateral projections. Two experienced investigators reviewed each coronary angiogram in a blinded fashion. In case of a lack of agreement between the two investigators, a third one was sought, and a conclusion was made.
Assessment of cardiac function
Two-dimensional transthoracic echocardiography (TTE) was performed twice, 2 days (2D) and 6 months (6M) after primary PCI, at rest in a left decubitus position, using a Vivid S5 ultrasound machine (GE, Solingen, Germany) equipped with the multi-frequency harmonic transducer 3Sc-RS (1.3–4 MHz). All measurements were carried out according to joint recommendations of the American Society of Echocardiography and European Association of Echocardiography [17] by one observer blinded to clinical and angiographic data. Images were recorded in the parasternal long axis, parasternal short axis, apical four-chamber, apical two-chamber, and apical long axis views of the LV. In apical four-chamber and apical two-chamber views, LV end-diastolic (LVEDV) and end-systolic (LVESV) volumes and ejection fraction (LVEF) were calculated according to the biplane Simpson method. The values of LVEDV and LVESV were subsequently indexed to the body surface area (LVEDVI, LVESVI [ml/m2]). The wall motion score index was calculated for IRA territory (IRA-WMSI) [18]. Based on the current recommendations [17], a 16-segment model for LV segmentation and classification of territories supplied by each coronary artery were used. Wall motion of each segment was analyzed individually by a trained physician and scored as normal or hyperkinetic – 1, hypokinetic – 2, akinetic – 3 and dyskinetic (or aneurysmatic) – 4. The function of each segment was validated in multiple views and the IRA-WMSI was expressed as the average value from all analyzed segments in the IRA territory.
Clinical outcomes
A 1-year clinical outcome included death, recurrent MI, and recurrent hospitalization due to symptoms of heart failure. Event-free survival after 1-year observation was defined as freedom from death, reinfarction, and repeated cardiovascular hospitalization.
Data were obtained from the hospital records and supplemented by direct and/or telephone interview with the patient. Cardiac function at the end of the study was assessed according to the New York Heart Association functional scale (NYHA). The NYHA functional class was determined by direct contact or telephone interview with patients.
Statistical analysis
Statistical analyses were performed with Statistica 6 (StatSoft, Inc). Continuous variables are expressed as a mean ± standard deviation or median (interquartile range) and categorical variables as a number (percentage). Continuous variables were first checked for normal distribution by the Shapiro-Wilk statistic and compared by ANOVA when normally distributed or by the Kruskal-Wallis test for non-normally distributed variables, both with appropriate post-hoc tests. Categorical variables were analyzed by χ2 or Fisher’s exact test. Pearson’s or Spearman’s rank correlation coefficients were calculated to test the association between two variables with a normal or non-normal distribution, respectively. Multivariate regression analysis was used to determine independent predictors of changes of LV function and remodeling. A p-value of less than 0.05 was considered statistically significant.
Results
Of 155 STEMI patients with a patent IRA at baseline, 19 (12.3%) patients with TIMI-2 flow (T2Res) and 85 (54.8%) patients with TIMI-3 flow (T3Res) achieved further STE resolution following PCI, 31 (20.0%) patients with TIMI-2 (n = 5) or 3 (n = 26) flow developed reSTE of ≥ 1 mm following PCI as compared with pre-PCI recordings (T23reSTE) and the last 20 (12.9%) patients with baseline TIMI-3 did not require PCI because of residual stenosis of less than 50% (T3noPCI).
Patients’ baseline and procedural characteristics are shown in Tables I and II. The studied groups did not differ in terms of demographic variables, cardiovascular risk factors, time of ischemia, laboratory results, IRA distribution or prehospital pharmacological regimen (Table I). Among the compared groups, there were also no significant differences in the thrombus burden expressed as TIMI thrombus grade (TTG) or distal embolization; however, incomplete TMGP-0/1 perfusion was more frequently observed in T2Res patients before PCI and in T2Res and T23reSTE patients after PCI as compared with remaining subgroups; therefore these patients required abciximab use the most frequently (Table II).
Table I
Baseline characteristics of studied patients
[i] Data are expressed as number (percentage) or median (interquartile range). MI – myocardial infarction, NYHA – New York Heart Association, PCI – percutaneous coronary intervention, T2Res – TIMI-2 flow with ST-segment resolution following PCI, T23reSTE – TIMI-2/3 flow with ST-segment re-elevation following PCI, T3noPCI – TIMI-3 flow without PCI, T3Res – TIMI-3 flow with ST-segment resolution following PCI.
Table II
Antithrombotic pharmacotherapy and invasive procedure
[i] Data are expressed as number (percentage). FMC – first medical contact, PCI – percutaneous coronary intervention, TIMI – thrombolysis in myocardial infarction, TMPG – TIMI myocardial perfusion grade, T2Res – TIMI-2 flow with ST-segment resolution following PCI, T23reSTE – TIMI-2/3 flow with ST-segment re-elevation following PCI, T3noPCI – TIMI-3 flow without PCI, T3Res – TIMI-3 flow with ST-segment resolution following PCI.
Changes of ST-segment elevation
Among the compared groups, there was no significant difference in the sum of STE in qualifying ECG (p = 0.10); however, there were significant differences of STE in ECGs recorded before (p = 0.005) and after PCI (p < 0.001) (Figure 1 A). Before PCI, STE was significantly higher in T2Res patients as compared with T3Res (mean difference: 4.0 mm, 95% CI: 0.7–7.3 mm) and T3noPCI (5.0 mm, 95% CI: 0.8–0.1 mm). After PCI, residual STE was significantly higher in T23reSTE as compared with T3Res (5.0 mm, 95% CI: 3.4–6.8 mm) and T2Res (2.3 mm, 95% CI: 0.5–5.2 mm). There were also significant differences of STRB (p = 0.008) and STRA (p < 0.001) (Figure 1 B). STRB was significantly lower in T2Res patients as compared with T3noPCI (36.8%, 95% CI: 8.2–65.4%). STRA was significantly lower in T23reSTE as compared with both T3Res (36.0%, 95% CI: 24.2–47.4%) and T2Res (22.8%, 95% CI: 6.4–39.1%).
Figure 1
Changes of ST-segment elevation and ST-segment elevation resolution in the studied patients
Data are shown as mean and standard deviation. Q, B, A – ECG recorded at first medical contact, before and after PCI, respectively. T2Res – TIMI-2 flow with ST-segment resolution following PCI, T23reSTE – TIMI-2/3 flow with ST-segment re-elevation following PCI, T3noPCI – TIMI-3 flow without PCI, T3Res – TIMI-3 flow with ST-segment resolution following PCI. *P < 0.05 vs. B-T2Res, **p < 0.001 vs. A-T23reSTE, ***p < 0.05 vs. A-T2Res, ****p < 0.05 vs. A-T23reSTE.

Evolution of LV systolic function
Among the compared groups, there were significant differences of LV ejection fraction 2 days (p = 0.001) and 6 months following PCI (p = 0.005) (Figure 2 A). LVEF2D was significantly lower in T23reSTE patients as compared with T3Res (mean difference: 7.4%, 95% CI: 2.5–12.3%). In 6-month observation, LVEF increase in T3Res (by 3.9 ±5.1%) and in T3noPCI (by 5.7 ±6.1%) patients was higher as compared with that in T23reSTE patients (0.2 ±7.0%, p < 0.05 for both) (Figure 2 B). At 6M, LVEF was significantly lower in T23reSTE as compared with T3Res (5.5%, 95% CI: 1.6–16.5%) and T3noPCI (3.3%, 95% CI: 6.4–39.1%). In T2Res patients, LVEF did not change significantly in 6-month observation.
Figure 2
Changes of global and infarct-related contractility of left ventricle
Data are shown as mean and standard deviation. 2D – measurements performed 2 days after PCI, 6M – measurements performed 6 months after PCI, LVEF – left ventricular ejection fraction, IRA WMSI – wall motion index score for infarct-related territory, T2Res – TIMI-2 flow with ST-segment resolution following PCI, T23reSTE – TIMI-2/3 flow with ST-segment re-elevation following PCI, T3noPCI – TIMI-3 flow without PCI, T3Res – TIMI-3 flow with ST-segment resolution following PCI. *P < 0.001 vs. 2D-T3Res, **p < 0.01 vs. 6M-T23reSTE, ***p < 0.05 vs. T23reSTE.
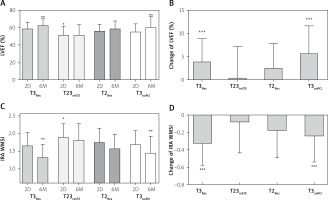
There were also significant differences in wall motion score index values calculated for infarct-related territory (IRA-WMSI) both 2 days (p = 0.03) and 6 months following PCI (p < 0.001) (Figure 2 C). The IRA-WMSI2D was significantly higher in T23reSTE patients as compared with T3Res (mean difference: 0.25, 95% CI: 0.02–0.49). In 6-month observation, IRA-WMSI improvement in T3Res (by 0.33 ±0.25) and in T3noPCI (by 0.24 ±0.29) patients was higher as compared with T23reSTE patients (0.08 ±0.35, p < 0.05 for both) (Figure 2 D). In T2Res patients, IRA-WMSI did not change in 6-month observation. At 6 months, differences in IRA-WMSI were more pronounced and IRA-WMSI was significantly higher in T23reSTE as compared with T3Res (0.49, 95% CI: 0.25–0.72) and T3noPCI (0.36, 95% CI: 0.04–0.68).
LV remodeling
There were no differences among the compared groups in terms of baseline LVEDVI (p = 0.89) and LVESVI (p = 0.08) (Figures 3 A, C). In 6-month follow-up, LVEDVI increased in T23reSTE by 6.6 ±12.6 ml/m2 and decreased in group T3Res by 3.8 ±9.7 ml/m2 and T3noPCI by 2.4 ±6.2 ml/m2 (for both p < 0.05 vs. T23reSTE) (Figure 3 B). In T2Res patients, LVEDVI did not change significantly in 6-month observation. Therefore, after 6 months, LVEDVI was significantly higher in T23reSTE as compared with T3Res (9.9 ml/m2, 95% CI: 0.6–19.3 ml/m2) (Figure 3 A).
Figure 3
Changes of left ventricular volume indexes
Data are shown as mean and standard deviation. 2D – measurements performed 2 days after PCI, 6M – measurements performed 6 months after PCI, LVEDVI – left ventricular end-diastolic volume index, LVESVI – left ventricular end-systolic volume index, T2Res – TIMI-2 flow with ST-segment resolution following PCI, T23reSTE – TIMI-2/3 flow with ST-segment re-elevation following PCI, T3noPCI – TIMI-3 flow without PCI, T3Res – TIMI-3 flow with ST-segment resolution following PCI. *P < 0.001 vs. 6M-T23reSTE, **p < 0.01 vs. 6M-T23reSTE, ***p < 0.05 vs. T23reSTE.
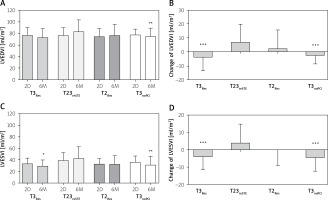
In 6-month follow-up, LVESVI increased in T23reSTE (by 3.8 ±10.8 ml/m2) patients, did not change significantly in T2PCI (by 0.1 ±9.0 ml/m2) patients, whereas it decreased in T3Res (by 4.2 ±7.2 ml/m2) and in T3noPCI (by 4.7 ±7.7 ml/m2) patients (for both p < 0.05 as compared with T23reSTE) (Figure 3 D). Finally, after 6 months LVESVI was significantly higher in T23reSTE as compared with T3Res (13.8 ml/m2, 95% CI: 5.8–21.9 ml/m2) and with T3noPCI (11.5 ml/m2, 95% CI: 0.5–22.4 ml/m2) (Figure 3 C).
Clinical outcomes
The time of hospitalization and the number of patients with symptoms of heart failure in the NYHA scale ≥ 2 after 12 months were similar in all groups (Table I). In 12-month follow-up, there were no deaths, 2 (1.3%) patients had recurrent MI and 9 (5.8%) required hospitalization due to symptoms of heart failure. There were no significant intergroup differences (Table I).
Predictive value of reSTE
The multivariate models for the recovery of LV function and LV remodeling are shown in Table III. Independent variables identified as associated (p < 0.2) with dependent variables in the univariate model (Tables I, II) were included in the multivariate regression models. Moreover, significant correlations between independent variables including LVEF2D and infarct territory (r = 0.31, p < 0.001), LVEF2D and the incidence of reSTE (r = 0.28, p < 0.001), LVEDVI2D and infarct territory (r = 0.19, p = 0.011) and LVESVI2D and infarct territory (r = 0.40, p < 0.001) were found. In final multivariate models, reSTE and baseline LVEF independently affected changes of LVEF with variance of R2 = 0.31 (p = 0.002) (Table III), whereas only reSTE independently predicted changes of both LVEDVI and LVESVI with variance of R2 = 0.35 and R2 = 0.36, respectively (p < 0.001 for both).
Table III
Independent predictors of changes of LVEF, LVEDVI and LVESVI
[i] IRA – infarct-related artery, LAD – left anterior descending artery, LVEF – left ventricular ejection fraction, LVEDVI – left ventricular end-diastolic volume index, LVESVI – left ventricular end-systolic volume index, reSTE – ST-segment re-elevation following PCI, 2D – measurements performed two days after PCI, R2 – variance.
Discussion
To our knowledge, the current study constitutes one of the infrequent reports regarding clinical relevance of reSTE following primary PCI in patients with spontaneously recanalized IRA. We demonstrated that reSTE was independently associated with both lack of improvement of global and infarct-related contractility and increase of LV volume in 6-month observation. Moreover, the procedure accompanied by reSTE was related to worse LV function and structure recovery as compared with patients with subsequent ST-segment resolution following PCI. Our findings provide evidence that myocardial injury reflected by reSTE is associated with the loss of beneficial effects of spontaneous IRA reperfusion on further LV remodeling.
Our study also provides some evidence regarding the mechanism of reSTE. We have shown that angiographically visible distal embolization occurred in around 10% of patients with reSTE and occurred in a similar proportion in the remaining analyzed groups and other studies [19, 20]. Moreover, we found no significantly different distribution of TIMI thrombus grades in the compared groups, which suggests a lack of unquestionable association between reSTE and angiographically evident residual epicardial thrombus. Simultaneously, the results of our study emphasize the role of impaired microvascular perfusion in patients with reSTE. Incomplete TMPG-0/1 reperfusion after PCI was the most frequent in patients with reSTE. Moreover, in about 30% of patients with reSTE, myocardial perfusion was deteriorated from baseline TMPG-2/3 to 0/1 following PCI. This observation is consistent with our previous findings [21] and reports on microvascular injury assessed with cardiac magnetic resonance in patients with reSTE [12]. It might be speculated that during balloon inflation, the components of ruptured plaque containing connective tissue elements and subendothelial matrix proteins are exposed to the bloodstream and initialize platelet activation, thrombin generation followed by thrombus formation and promote vasospasm. Prothrombotic material protruding through the struts during stent implantation together with the already present intraluminal thrombus may lead to epicardial blood flow deterioration [22] and microvascular plugging [23]. Identification of the key factor involved in myocardial perfusion deterioration in patients with reSTE after spontaneous reperfusion requires further studies with the use of optical coherent tomography. Interestingly, in our study reSTE was associated with a similar systemic inflammatory response as measured by white blood cell count and hsCRP level. In contrast, a previous study reported that CRP concentration in STEMI patients with reSTE was higher, reflecting more intensified reperfusion injury [11].
In our group, unfavorable echocardiographic findings related to reSTE did not affect the duration of hospitalization or 1-year clinical outcomes. Research conducted so far regarding the impact of reSTE following PCI on LV function concerns mainly patients with initially occluded IRA. In a small observational study including 41 STEMI patients, Weaver et al. [12] found a significant relationship between reSTE and the extent of myocardial injury assessed by contrast-enhanced cardiac magnetic resonance. In turn, Okuda et al. [11] revealed that reSTE in patients who underwent both primary PCI and thrombolysis was associated with larger infarct size and poorer LVEF and LVEDVI at 6 months. A recently published subanalysis of the large randomized CIRCUS trial [9] showed no unfavorable effects of reSTE on LV contractility and remodeling at 1-year follow-up. Patients with and without the reSTE primary endpoint composed of all-cause mortality and heart failure were found with similar proportion (19.2 vs. 19.8%, p = 0.887) at 1 year. It should be emphasized that only patients with a large anterior wall infarction were included in this study, which may mask the effects associated with reSTE. Our findings suggest that the incidence of reSTE following PCI in a patent IRA produces the risk of LVEF deterioration on average by 4.4% and the risk of LVEDVI or LVESVI increase by 7 or 8.5 ml/m2, respectively.
In the context of high prevalence of impaired tissue-level microvascular perfusion in patients with reSTE following PCI in a spontaneously reperfused IRA and its unfavorable long-term LV consequences, implementation of appropriate preventive and therapeutic strategies seems to be a key goal. As was documented in the DEFER-STEMI study [24], deferred PCI in selected STEMI patients reduced no-reflow, distal embolization and other thrombotic complications as compared with immediate stenting. However, other studies provided considerably less optimistic conclusions. In DANAMI 3-DEFER [25], deferred PCI did not reduce the occurrence of death, heart failure, MI or subsequent revascularization as compared with conventional PCI. Moreover, routine deferred stenting was associated with a higher frequency of target vessel revascularization. A recent meta-analysis summarizing prior randomized trials showed that the deferred-stenting strategy did not reduce the occurrence of no-reflow, death, MI or repeated revascularization despite improved LVEF in long-term observation [26]. Based on these findings, routine deferred stenting is not recommended in the current European Society of Cardiology (ESC) guidelines [27]. Another intensively investigated strategy is thrombus aspiration. The two recent randomized trials TASTE [28] and TOTAL [29] showed no clinical benefit of routine thrombus aspiration in STEMI patients, suggesting possible increased risk of stroke. A meta-analysis of the trials TAPAS [30], TASTE and TOTAL finally confirmed that routine thrombus aspiration in STEMI patients did not improve clinical outcomes [31]. This meta-analysis, however, highlighted the group with high thrombus burden in which use of aspiration thrombectomy was associated with lower cardiovascular mortality (HR = 0.80, 95% CI: 0.65–0.98) but also with increased risk of stroke or transient ischemic attack (OR = 1.56, 95% CI: 1.02–2.42). Based on these data, routine thrombus aspiration is also not recommended in current ESC guidelines but in cases of large residual thrombus burden, after opening of the IRA, thrombus aspiration may be considered [27]. The current guidelines of ESC, however, permit the application of IIb/IIIa inhibitors as bailout therapy in the case of large thrombus, slow- or no-reflow, and other thrombotic complications [27]. Furthermore, a recently published study demonstrated the beneficial effect of early intracoronary administration of nicorandil on microcirculation damage [32]. Other strategies aimed at reduction of reperfusion injury, including cyclosporine, which activates mitochondrial potassium channels, failed to improve clinical outcomes and prevent LV remodeling [33], despite previous experimental studies indicating its beneficial effects on infarct size and microvascular obstruction [34].
Our study has several limitations. First, the sample size and the number of clinical adverse events are not large enough to draw clinical conclusions. Second, left ventricular remodeling was assessed by echocardiography, although cardiac magnetic resonance is a more accurate and objective method. Third, all participants were meticulously studied with serial ECGs only in the periprocedural period. Subsequent ECGs, including 60–90 min after PCI, were not prospectively recorded. Fourth, platelet reactivity [35], fibrin clot properties [36] and intracoronary thrombi [37] were not analyzed. However, these laboratory tests were beyond the main scope of this study.
Conclusions
ReSTE following PCI in patients with spontaneously recanalized IRA is associated with a lack of improvement of LV contractility and subsequent LV remodeling and therefore abolishes the beneficial effects of spontaneous reperfusion. Further studies on therapies to prevent reSTE following primary PCI are necessary.








