Introduction
Hepatic dysfunction, also known as liver dysfunction, is a medical condition characterized by impaired liver function. This condition manifests as a chronic or acute subclinical cellular disturbance but can progress into life-threatening hepatic failure with multiple organ failure when left untreated [1]. Research has shown that in the United States alone, more than 100 million people live with some form of hepatic dysfunction; however, only 4.5 million adults have been diagnosed with this condition [2]. The most common aetiologies of liver dysfunction include viral infections (hepatitis A, B, C, D, and E), alcohol abuse, non-alcoholic fatty liver disease, autoimmune hepatitis, medications, toxins, and genetic conditions such as haemochromatosis, Wilson’s disease, and α1 antitrypsin [3].
Because not all liver disease patients are suitable for transplantations and there is a shortage of grafts [4, 5], the need for liver support therapies to stabilize hepatic functions or enable bridging to liver transplantations has been enhanced. In this regard, extracorporeal albumin dialysis (ECAD) has been a valuable supplement to standard medical therapy (SMT). The commonly used ECAD therapies are Single-Pass Albumin Dialysis (SPAD), Prometheus, and Molecular Adsorbent Recycling System (MARS). MARS is currently the most common ECAD that combines dialysis with adsorption techniques to remove water-soluble and protein-bound toxins in the bloodstream of hepatic dysfunction patients. Several clinical trials have shown that MARS is a feasible tool for reducing bilirubin levels, improving hepatic encephalopathy (HE) and enhancing haemodynamic stability [6–8]. However, large clinical trials have shown that this technique has no survival benefit and has potential complications such as coagulopathy and hypotension [8]. On the other hand, SPAD is a liver support therapy that utilizes a high-flux dialysis membrane to effectively capture and remove albumin-bound toxins. Clinical trials have shown that SPAD is associated with the reduction of bilirubin levels and improvement of the patient’s clinical condition (HE) [9]. Finally, Prometheus is a liver support therapy combining fractioned plasma separation and adsorption (FPSA) with flux dialysis to eliminate a wide range of toxins while simultaneously replacing essential molecules.
Although these ECAD devices have been incorporated to supplement SMT, their efficacy and safety are often questioned. Therefore, we carried out the current systematic review to summarize the evidence about the effectiveness of MARS, SPAD, and Prometheus in the reduction of albumin-bound and water-soluble substances, improvement of HE, reduction of mortality, and their safety among patients with various liver-related disorders.
Methodology
Information sources and searches
The PubMed, Medline, Cochrane Library, Web of Science, and Google Scholar electronic databases were extensively searched for all randomized trials published from inception until October 2023. In addition, bibliographies of potential articles were scrutinized for more studies. During the database search, the advance option was employed to search for articles with the following MeSH terms and keywords: (albumin dialysis OR Extracorporeal albumin dialysis OR Prometheus OR molecular adsorbent recycling system OR MARS OR Single-Pass Albumin dialysis OR liver hemodialysis OR Fractioned Plasma Separation and Adsorption) AND (hepatic dysfunction OR liver dysfunction OR liver disease OR liver failure OR Acute-on-chronic liver failure OR acute liver failure). Furthermore, grey literature and exact or close duplicates were eliminated because they would interfere with the scientific research purpose of the present study.
Eligibility criteria
Two impartial reviewers examined potential studies that adhered to the predefined eligibility criteria. Studies were eligible for inclusion if they were randomized trials published in English, comprised adult human patients with liver disorders, and compared the safety or efficacy of SPAD, MARS, and Prometheus to SMT or each other. On the other hand, studies that did not match these criteria or were designed as case reports, conference abstracts, systematic reviews, letters to the editors, or did not have comparative groups were excluded. All the discrepancies during this process were amicably resolved by consulting a third reviewer.
Data extraction
Two reviewers independently abstracted the data required for review and analysis into separate Excel files. In case of disparities in abstracted data, the 2 reviewers resolved their differences via constructive debates or by consulting a third reviewer if a compromise was not met. The data extracted by these reviewers included author ID (surname of the primary author and the year of publication), study design, study period, location of the study (country), pertinent characteristics of the enrolled patients (sample size, gender distribution, and the underlying liver-related disorder(s)), interventions (albumin dialysis systems used and comparator), follow-up duration, and outcomes.
The outcomes were categorized as either safety or efficacy endpoints. The efficacy endpoints comprised changes in total bilirubin, bile acids, ammonia, HE improvements, and mortality outcomes. On the other hand, safety outcomes encompassed all the adverse events witnessed after treatment. Improvement in HE was defined as the rate of individuals who had at least 2-grade improvement in West Haven grade of HE from baseline.
Quality appraisal
All the studies in the present systematic review were randomized trials; therefore, the potential risk of bias was assessed by 2 independent reviewers using Cochrane’s risk of bias tool embedded within the Review Manager software. The risk of bias was evaluated according to 5 domains, i.e. selection, attrition, performance, reporting, and other biases. For each domain, the low risk of bias was assigned for a fully addressed criterion, while high and unclear risk of bias was assigned to criteria not addressed or with insufficient information, respectively.
Data synthesis
Review Manager software (version 5.4.1) was used to conduct all meta-analytic data analyses in the present review. The DerSimonian and Laird random effects model was used in these analyses to pool the estimated weighted effect sizes. Moreover, for dichotomous outcomes, the overall effect sizes were calculated using risk ratio (RR) and their corresponding 95% confidence intervals (CI), while for continuous outcomes, the effect sizes were calculated using the mean differences (MD). The heterogeneity between study outcomes was calculated using the I2 and χ2 statistics, for which values of > 50% and < 0.10, respectively, were considered significant [10]. In cases where data were presented as median, range, and interquartile ranges, the means and standard deviations were calculated according to the formula described by Hozo et al. [11]. Whenever possible, subgroup analyses were carried out according to the ECAD modality.
Results
Study selection
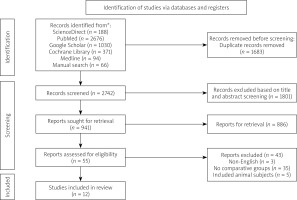
Our preliminary database search revealed 4359 viable articles with the specified MeSH phrases and keywords. Additionally, the in-depth review of reference lists uncovered 66 potential records. After a duplicate screening of these articles, 1683 regarded as exacts or nearly identical duplicates were omitted. Out of the remaining 2742 records, 1801 were discarded because they violated the title and abstract screening criteria, while 886 were not retrieved because they were either conference abstracts, case reports, ongoing trials, in vitro studies, observational studies, letters to the editors, or systematic reviews and meta-analyses. Finally, only 12 papers were accepted for review and analysis, and the remaining 43 were discarded based on the following exclusion criteria: 3 were published in different languages, 35 did not have comparative groups, and 5 included animal subjects (Figure 1).
Summary of study characteristics and risk of bias assessment
Characteristics of individual studies are summarized in Table I [12–20]. The 12 trials spanned 21 years and included 653 patients with varied liver-related disorders. Nine of these trials were performed in individual countries, and 3 were in multiple countries. In addition, 6 trials compared MARS treatment to SMT, one compared Prometheus to SMT, 2 compared MARS to SPAD, one compared MARS to Prometheus, and 2 compared both MARS and Prometheus to SMT.
Table I
Study characteristics
[i] SPAD – Single-Pass Albumin Dialysis, MARS – Molecular Adsorbent Recycling System, SMT – Standard Medical Therapy, NR – not reported, RCT – randomized controlled trial, HE – hepatic encephalopathy, ALD – alcoholic liver disease, NAFLD – non-alcoholic fatty liver disease, PBC – primary biliary cirrhosis, HDF – haemodiafiltration.
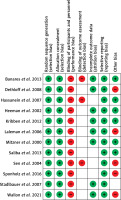
The risk of bias is summarized in Figure 2. The assessment shows that all the trials had a low risk of bias under the selection domain (i.e. random sequence generation and allocation concealment). Furthermore, all the studies were considered to have a high risk of performance because it was not possible to blind both patients and personnel to the treatment. Under the other bias domain, studies were considered to have a high risk of bias if they were carried out in single centres.
Efficacy of ECAD in the reduction of Albumin-bound and water-soluble substances
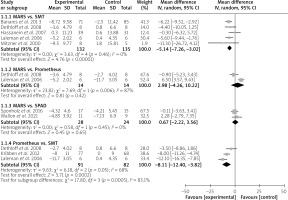
In the current systematic review, albumin-bound toxins were represented by total bilirubin and bile acids. Subgroup analysis of data from 5 included studies showed that, compared to SMT, the removal of bilirubin was more pronounced with the use of MARS (MD = –5.14 mg/dl; 95% CI: –7.26 – –3.02; p < 0.00001) (Figure 3). Similarly, data from 3 trials revealed that Prometheus is more efficient than SMT in removing bilirubin (MD = –8.11 mg/dl.; 95% CI: –12.40 – –3.82; p = 0.0002) (Figure 3). However, our subgroup analysis showed that MARS has a comparable effect in the reduction of bilirubin as Prometheus (MD = 2.98 mg/dl; 95% CI: –4.26 – 10.22; p = 0.42) and SPAD (MD = 0.67 mg/dl; 95% CI: –2.22 – 3.56; p = 0.65) (Figure 3).
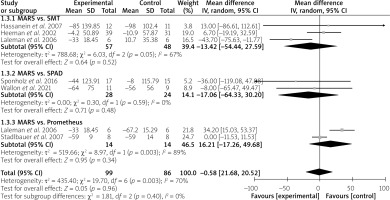
On the other hand, a subgroup meta-analysis revealed that MARS has a similar effect in the reduction of total bile acids as SMT (MD = –13.42 µmol/l; 95% CI: –54.44 – 27.59; p = 0.52), SPAD (MD = –17.06 µmol/l; 95% CI: –64.33 – 30.20; p = 0.48) and Prometheus (MD = 16.21 µmol/l; 95% CI:–17.26 – 49.68; p = 0.34) (Figure 4). However, the variation between these outcomes was significant (I2 = 67% and 89% for MARS vs. SMT and MARS vs. Prometheus, respectively).
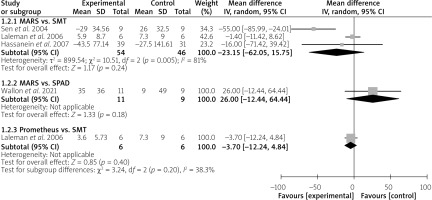
In addition, the removal of water-soluble substances was assessed through ammonia levels. The subgroup analysis demonstrated that MARS has a comparable effect in the reduction of ammonia to that of SMT (MD = –23.15 µmol/l; 95% CI: –62.05 – 15.75; p = 0.24) and SPAD (MD = 26 µmol/l; 95% CI: –12.44 – 64.44; p = 0.18) (Figure 5). Similarly, Prometheus did not significantly reduce ammonia levels compared to SMT (MD = –3.70 µmol/l; 95% CI: –12.24 – 4.84) (Figure 5).
Efficacy of ECAD on improvement of HE
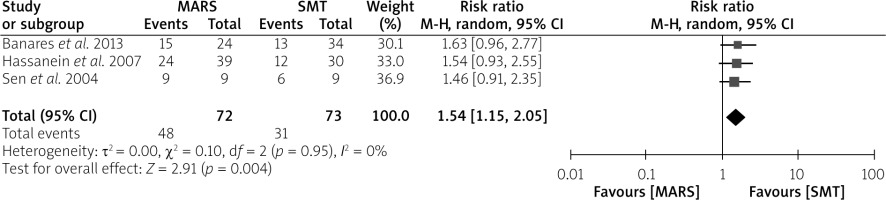
Although we aimed to analyse the effect of all ECAD systems on HE improvement, only 3 trials that compared MARS to SMT reported the effect on HE as one of the endpoints. The pooled analysis of data from these trials revealed that HE was significantly improved by the MARS device as opposed to the SMT (RR = 1.54; 95% CI: 1.15–2.05; p = 0.004) (Figure 6).
Efficacy of ECAD on mortality outcomes
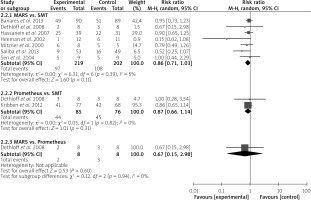
In the subgroup analyses, MARS did not result in a significant reduction in the risk of mortality compared to SMT (RR = 0.86; 95% CI: 0.71–1.03; p = 0.11) or Prometheus (RR = 0.67; 95% CI: 0.15–2.98; p = 0.60) (Figure 7). Similarly, albumin dialysis with Prometheus resulted in an insignificant reduction in mortality risk compared to SMT (RR = 0.87; 95% CI: 0.66–1.14; p = 0.31) (Figure 7).
Safety analysis
The adverse events reported in each study were highly inconsistent. However, we managed to group these adverse events as respiratory, haematological, or bleeding complications. Respiratory complications comprised pulmonary infection/pneumonia, respiratory failure, pulmonary bleeding, aspiration, and dyspnoea. On the other hand, haematological complications included leukopaenia/pancytopaenia and thrombocytopaenia, while bleeding complications included any bleeding events and coagulopathy. A meta-analysis of these complications revealed that MARS and Prometheus did not have any significant decrease in adverse events compared to SMT (Table II).
Table II
Summary of adverse events after liver support therapies
Discussion
This systematic review and meta-analysis summarizes the efficacy and safety of MARS, SPAD, and Prometheus in patients with hepatic dysfunction. The use of MARS and Prometheus was associated with a considerable reduction in total bilirubin levels compared to SMT. However, the reduction of bilirubin, bile acids, and ammonia between the 3 ECAD systems did not differ statistically, suggesting that MARS is as effective as Prometheus and SDAD in the removal of albumin-bound toxins and water-soluble substances. Moreover, the adverse effects witnessed after albumin dialysis with MARS and Prometheus were similar to those observed after SMT. Therefore, MARS and Prometheus are safe liver haemodialysis treatments.
The significant reduction in bilirubin levels after MARS and Prometheus treatment agrees with a previous systematic review of patients with acute and acute-on-chronic liver failure [21]. Furthermore, our analysis has shown that MARS is as effective as SPAD and Prometheus in reducing bilirubin levels. However, the heterogeneity observed in the analyses of MARS compared with Prometheus was significant, suggesting a variation in outcomes. Laleman et al. found that in patients with alcoholic cirrhosis superimposed with alcoholic hepatitis, Prometheus treatment had a more pronounced reduction of total bilirubin levels than MARS [15]. This significant difference can be attributed to the fact clearance of albumin-bound substances is dependent on the dialysate flow rate [22]. In that trial, the Prometheus flow rate was higher than that of MARS (300 ml/l vs. 200 ml/l, respectively) [15]. Another possibility for the significant difference is the bilirubin concentrations before treatment. In that trial, patients randomized to the Prometheus group had higher bilirubin levels than those in the MARS group (29.7 mg/dl vs. 21.8 mg/dl, respectively) [15]; hence, the clearance of these albumin-bound substances was likely to be higher in the Prometheus group.
Although bilirubin has been routinely used to assess the removal of albumin-bound substances, other parameters, such as bile acids, might be of greater clinical importance in defining excretory liver dysfunction. First, toxic bile acids are likely to induce hepatocyte and biliary epithelial cell necrosis [23]; hence, they are of prognostic importance [24, 25]. Furthermore, research suggests that toxic bile acids can result in remote organ dysfunction such as cirrhotic cardiomyopathy [26] and reduced systemic vascular resistance [27]. Our analysis found that MARS did not significantly reduce total bile acids compared to SMT, SPAD, or Prometheus. However, the variation compared to SMT and Prometheus was significant, suggesting highly inconsistent outcomes. In their research, Laleman et al. found that MARS had a significantly higher bile acids clearance than SMT. However, the clearance was more pronounced with the Prometheus device than the MARS device [15]. The difference observed in this study can be attributed to the conceptual differences between the devices. In contrast, Standlbeur et al. found no significant difference in the absolute reduction of total bile acids between the MARS and Prometheus devices [19]. However, they found that in the Prometheus group, the reduction ratio for cholic acid (CA) was slightly higher than that of chenodeoxycholic acid (CDCA). Furthermore, the plasma clearance of bile acids was studied over a 6-hour period, showing that at the beginning of treatment bile acid clearance was nearly identical between the Prometheus and MARS groups and within the range of previously published data [28, 29].
During liver dysfunction, acute or chronic renal insufficiency is a commonly observed complication. Therefore, the removal of other water-soluble substances, such as creatinine, during ECAD is of great importance. In their research, Banares et al. found that among patients with acutely decompensated cirrhosis, MARS treatment was associated with a significant reduction in serum creatinine than SMT (–20.04% vs. –6.43%; p = 0.022) [6]. This finding is consistent with what was documented in other studies [15, 16]. However, the research by Sen et al. [17] suggests that patients in the SMT group had a higher reduction in creatinine levels than those in the MARS group (from 88 to 86.5 µmol/l vs. 113 to 62.5 µmol/l, respectively). This finding can be explained by the fact that patients treated with SMT had higher creatinine levels before treatment than those treated with MARS. On the other hand, a prospective randomized trial of patients presenting acute liver failure demonstrated that MARS has a more pronounced efficacy in clearing water-soluble substances (i.e. creatinine and urea) than SPAD [18]. This improved efficacy of MARS may be due to the differing dialysate flow rates between devices (2000 ml/h vs. 700 ml/h, for MARS and SPAD, respectively) [19]. However, even after treating patients with SPAD and MARS of equal dialysate parameters, Wallon et al. found that MARS significantly reduced creatinine levels more than SPAD (p < 0.0001) [20]. Therefore, this clinical endpoint needs to be evaluated in more randomized trials to effectively define the role of SPAD in clearing creatinine compared to MARS.
Regarding HE improvement, we found that ECAD with MARS was more efficient than SMT in improving HE. Furthermore, Hassanein et al. reported that although MARS treatment was delayed by 5.25 h after randomization, the time to HE improvement was significantly shorter in the MARS group than in the SMT group (p = 0.044) [7]. This improvement in HE with MARS treatment agrees with previously published data [28, 30–32] and can be attributed to various factors. First, HE improvement can be explained by a reduction in ammonia concentrations. However, like in previous research [30, 33], HE improved despite insignificant ammonia change. This finding suggests that improvement in HE after MARS treatment is independent of hyperammonaemia improvement. Another factor believed to be important in HE pathogenesis is inflammation [34]. Therefore, the reduction of proinflammatory cytokines might have resulted in HE improvement. Oxidative stress is also believed to be vital in HE pathogenesis [35–37] because of its effects on mitochondria, membrane phospholipids oxidation, and various enzymes involved in metabolism [37]. Furthermore, the reduction of plasma nitrate and nitrite (NOx) may be considered as an underlying mechanism in HE improvement. Although the role of NO in HE remains unclear, there is emerging evidence that nitridation of critical proteins might contribute to altering their function or cause injury through nitrotyrosine formation.
Although our meta-analysis demonstrated a significant improvement in HE after MARS treatment, the subgroup analysis suggests that MARS has no mortality benefit in patients with hepatic dysfunction. This finding is highly paradoxical and needs further randomized trials to establish the mortality benefit of MARS. Furthermore, our analysis implied that Prometheus does not offer any significant reduction in mortality compared with SMT. This lack of survival improvement after albumin dialysis with either Prometheus or MARS can be attributed to several factors. One of the potential explanations is that the beneficial effects may have been counterbalanced by the complications associated with these procedures. This is evident in our study, where Prometheus and MARS have similar adverse events to SMT alone. Another possibility is that all the trials used for the mortality analysis were statistically underpowered to analyse this hard clinical endpoint, especially in clinical settings where the underlying comorbidities and severity of illness are predominant predictors of clinical outcomes. Finally, the number of ECAD sessions may have influenced the effect of these devices on mortality outcomes. Kribben et al. reported that the duration or number of FPSA sessions was insufficient for any survival benefit to be observed (average of 8 sessions) [14].
The present systematic review is subject to several limitations that ought to be considered while interpreting our findings. First, significant heterogeneity was observed in some of the meta-analyses. However, this heterogeneity in outcomes was expected given that the present systematic review pooled outcomes from studies with diverse methodologies, severity of liver dysfunction, underlying liver diseases, follow-up duration, and dialysate flow rates. Therefore, the use of a random-effects model to pool the outcomes was justified. Secondly, in all trials, double blinding was not possible; hence, it is possible that this bias was transferred to our assessment of HE improvement. Thirdly, due to limited trials on SPAD, we could not conclude the safety of SPAD and its impact on mortality outcomes among hepatic dysfunction patients. Finally, our eligibility criteria did not accommodate the inclusion of articles published in different languages and those with abstracts only, meaning that data from these articles that would have been used to improve the statistical power of meta-analysis were eliminated.
Conclusions
In patients with liver-related disorders, MARS, SPAD, and Prometheus are equally effective in reducing albumin-bound and water-soluble substances, as indicated by changes in bilirubin, bile acids, and ammonia levels. Moreover, there is evidence suggesting that Prometheus may be more beneficial than MARS in the removal of toxic bile acids, while MARS with similar dialysate flow parameters as SPAD may increase the reduction of water-soluble substances such as creatinine. However, these findings prompt the conduct of more high-quality randomized trials. In addition, MARS and Prometheus have similar adverse events as SMT; therefore, these ECAD devices are safe treatments for patients with hepatic dysfunction. Furthermore, MARS is associated with a considerable improvement in HE without any significant improvement in survival outcome. Therefore, this calls for more randomized trials that evaluate the survival benefits of ECAD devices in patients with hepatic dysfunction.