Introduction
Gastric cancer is the fifth most common cancer worldwide. It is the fourth most commonly occurring cancer in men and the seventh most commonly occurring cancer in women. There were over 1 million new cases in 2018 [1]. In the past 60 years, the incidence of gastric cancer has decreased significantly compared with previous data. But it is undeniable that gastric cancer is still a very serious disease and that it is a very serious problem; the cause of gastric cancer is not very clear, but there is no doubt that some people are more likely to suffer from this disease than others [2].
The most commonly used staging system for gastric cancer is the Tumor/Node/Metastasis (TNM)/American Joint Committee on Cancer (AJCC) system. Successive editions of this staging system have been released reflecting the advances in our knowledge of gastric cancer prognosis and treatment approaches [3]. The staging systems we used in this study are AJCC TNM, 7th ed. (2010-2015) and AJCC Stage Group, 7th ed. (2010-2015).
Aim
In order to explore the cause of gastric cancer, we analysed the data from the SEER database and obtained some valuable information. The contents of our study are as follows: the effects of various physical indicators on the incidence of gastric cancer, the differences of different type of surgery for patients with different stages, and the differences of the different type of surgery for the same operation.
Material and methods
The cases evaluated in this analysis were extracted from the SEER-18 registry [4]; in order to achieve this, SEER*Stat software (Version 8.3.5) was used. The date of the SEER data submission was November 2018.
Selection of the study cohort
Patients were screened from the SEER database between 2010 and 2015. In order to identify patients with primary gastric tumours, “Stomach” in the CS Schema-AJCC 6th Edition category was selected. Patients with primary tumours are identified by screening for “Yes” in the attribute first malignant primary indicator. Cases with deficient information about their marital status at diagnosis, insurance recode (2007+), grade, RX Summ-SurgPrimSite (1998+), CS tumour size (2004–2015), AJCC 7th stage, and survival were excluded.
Data collection
Information extracted for each patient included sex, race recode (W, B, AI, API), age at diagnosis, marital status at diagnosis, insurance recode (2007+), grade, RX Summ--Surg Prim Site (1998+), CS tumour size (2004–2015), first malignant primary indicator, survival (months), vital status recode (study cut-off used), 7th edition T, N, and M stages, and AJCC 7th edition stage group.
Statistical considerations
In this study, Kaplan-Meier analysis and log-rank testing were used for survival comparisons (including both overall survival and staging survival) according to the AJCC pathological stage. The cox proportional hazard model was conducted to produce multivariate analyses. A score of 77.6% is obtained by C-index calculation of the Cox model, and the score of the AUC is 77.0%, which shows that the model is statistically significant. All statistical analyses were performed in Python (Python3.7) and R language (R version 3.6.3).
Results
Patients’ characteristics
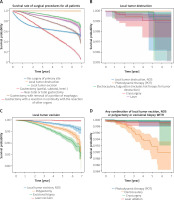
A total of 10,050 patients with surgically treated primary gastric tumour were identified in the period from 2010 to 2015 and were included in the analysis (Figure 1). In the dataset, gastrectomy (partial, subtotal, hemi-) represented half of the cases (50.5%), local tumour destruction (0.1%), local tumour excision (7.3%), near-total or total gastrectomy (11.6%), gastrectomy with removal of a portion of the oesophagus (N = 18.9%), gastrectomy with resection in continuity with the resection of other organs (10.9%), surgery NOS (0.4%), and gastrectomy NOS (0.3%). The most frequent age group was 40–69 years (56.3%), followed by the group of > 69 years (39.9%), and the age group < 40 years (3.8%). White race comprised 66.1%, black race represented 13.7%, Asian or Pacific Islander racial groups represented 19.5%, and the American Indian/Alaska Native racial groups represented 0.7%. The distribution of patients according to AJCC stages and type of surgery is shown in Table I.
Figure 1
Study enrolment of 14,507 primary gastric cancer records present 10,050 patients who had had surgery were included in the analysis
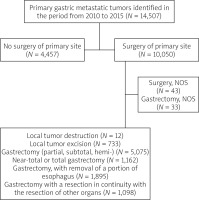
Table I
Baseline characteristics of included patients in the cohort (N = 10,050)
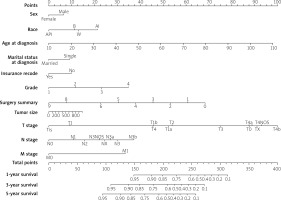
Multivariable prediction model based on cox regression model
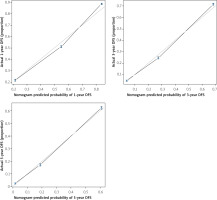
The data were analysed by the cox regression model, and the prediction model with the reference value was obtained (C-index score 77.6%, AUC score 77.0%). Some conclusions can be drawn from the nomogram of the cox regression model (Figure 2). It can be concluded from the calibration curve (Figure 3) that the age of diagnosis, histological grade, and the type of surgery have a great impact on the survival rate of patients. In the following, we will study the choice of surgical type.
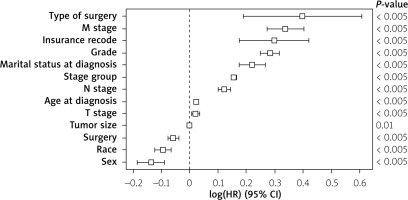
Multivariate analysis for factors affecting primary gastric cancer survival was conducted (evaluating the following factors: sex, race, age at diagnosis, marital status at diagnosis, insurance recode, grade, surgery, tumour size, survival months, T stage, N stage, M stage, and type of surgery). The following factors were associated with better primary gastric cancer survival (sex, race, age at diagnosis, marital status at diagnosis, insurance recode, grade, surgery, survival months, T stage, N stage, M stage, and type of surgery) (p < 0.005) (Figure 4).
Survival outcomes according to AJCC 7th ed. and type of surgery
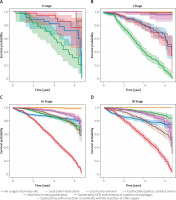
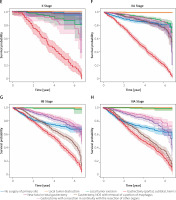
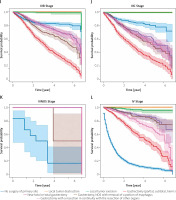
Overall, primary gastric cancer survival was compared according to both AJCC 7th ed. and type of surgery. Log-rank testing with pairwise comparisons between all different stages was conducted. In all the stage groups, except for the comparison between stages 0, I, II, and III NOS (Figures 5 A, B, E, K) there are significant (p < 0.005) differences among different stages. Part of the legend is shown in Figure 5. From the results of the Kaplan-Meier analysis, it can be seen that in the 0 stage group, the survival of patients who received gastrectomy was better, and in patients who received local tumour excision it was the worst (Figure 5 A). The same thing happened in the I stage group (Figure 5 B). In the staging group except 0, I, IIINOS and IV stage group (Figures 5 C–J), the survival of patients receiving gastronomy (partial, subtotal, hemi-) was the worst, while the survival of patients who received local tumour destruction and local tumour excision was the best. In the NOS III and IV stage groups (Figures 5 K, L), the survival was the worst among patients who did not undergo surgery.
It is noteworthy that due to the small amount of data in the 0 group (0.3%, Figure 5 A) and the III NOS group (0.02%, Figure 5 K) their importance is not high, thus affecting the overall value. More detailed studies on how surgical methods affect survival benefits will be described in later sections.
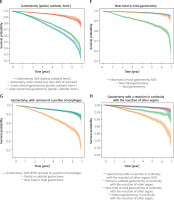
Survival outcomes according to the type of surgery and surgical methods
There was no significant difference among different groups except the local tumour destruction group (p = 0.77, Figure 6 B) and local tumour excision group (p = 0.29, Figure 6 C), but there was a significant difference in other groups (p < 0.005). In the 3-year survival, the survival among the “no surgery of primary site” group was the worst, but that of the gastrectomy (partial, subtotal, hemi-) group was the worst when it was greater than the 3-year survival (Figure 6 A). For the patients who had local tumour destruction surgery, the survival of each surgical method was roughly the same (Figure 6 B). In the local tumour excision surgery, the survival of the laser excision group was the best, while among the excisional biopsy group it was the worst (Figure 6 C). For local tumour excision surgery, except laser excision, the operation method has little effect on survival, but among the electrocautery group it was worse (Figure 6 D). In gastrectomy (partial, subtotal, hemi-) surgery, the survival of gastrectomy, NOS (partial, subtotal, hemi-), and lower (distal) gastrectomy (partial, subtotal, hemi-) group was worse than the antrectomy, lower (distal-less than 40% of stomach) and upper (proximal) gastrectomy (partial, subtotal, hemi-) group (Figure 6 E). Survival of the total gastrectomy group was the worst in the near-total or total gastrectomy surgery groups (Figure 6 F), and the survival of partial or subtotal gastrectomy was the worst in gastrectomy, with removal of a portion of oesophagus surgery (Figure 6 G). In the gastrectomy with a resection in continuity with the resection of other organs surgery, the survival of gastrectomy with a resection in continuity with the resection of other organs, NOS and radical gastrectomy, in continuity with the resection of other organs group was the best, and near total or total gastrectomy, in continuity with the resection of other organs was worse, while partial or subtotal gastrectomy, in continuity with the resection of other organs group was the worst (Figure 6 H).
Discussion
The incidence of gastric cancer has been steadily declining since the early part of the 20th century. However, due to the increase in life expectancy, the number of patients with gastric cancer continues to increase, especially among the elderly [5]. However, there are few studies on the choice of surgery for gastric cancer. Through 14,507 items of patient data from the SEER database, we used Cox multivariate regression model to explore the factors that affect the survival status of patients with gastric cancer, and came to the conclusion that age is the most important factor affecting the survival of patients, followed by the type of surgery, and histological grade, but these factors can not affect the survival of patients alone. For instance, advancing age is not an independent predictor of mortality following gastric surgery. Non-elective admission, pre-existing hypertension, valvular disease, and anaemia independently predicted increased morbidity and mortality following gastric surgery and should be carefully considered in surgical planning and counselling [6–8]. In this process, we also found some interesting results. For example, men are at a higher risk of developing gastric cancer than women; this result is consistent with the mainstream research showing that androgen receptor may be responsible for the gender disparity in gastric cancer [9]. Race also affects the prognosis of patients with gastric cancer to some extent; the same conclusion was drawn from other studies [10, 11]. Marital status and health insurance status also affect the prognosis of patients to some extent. The above conclusions are drawn from the nomogram (Figure 2). According to the type of surgery and the AJCC 7th ed. stage of the patients, the group experiment was carried out, and the Kaplan-Meier model was used to compare the survival rate of the patients, so as to judge the effect of the type of surgery on the survival benefit. The most striking conclusion is that gastrectomy (partial, subtotal, hemi-) is the worst type of surgery, especially in patients with intermediate and advanced gastric cancer. Gastrectomy NOS (partial, subtotal, hemi-) is the worst surgical method in patients with gastric cancer who undergo gastrectomy (partial, subtotal, hemi-). Three surgical methods: partial gastrectomy, Billroth I, and Billroth II, are included in the gastrectomy NOS (partial, subtotal, hemi-) group. Because there are no detailed records of Billroth I and Billroth II in the SEER database, it is generally believed that the Billroth II method of anastomosis is associated with higher rate of early postoperative complications, and the Billroth I method should be the first choice after a distal gastrectomy as long as the anatomic and oncological environment of an individual patient allows it [12]. In other radical operations, the effect of gastrectomy with a resection in continuity with the resection of other organs is the best, but the effect of AJCC 7th ed. V stage is the worst except Gastrectomy (partial, subtotal, hemi-). Among the conclusions of the study, there are also some that are contrary to the existing research conclusions. For example, near-total gastrectomy is not a better option for upper-third early gastric cancer than total gastrectomy [13], but in this study, the survival benefit of near-total gastrectomy was better than that of total gastrectomy (Figure 6 F). In palliative surgery, local tumour destruction and local tumour excision are both safe and effective treatments for early gastric cancer, and it is useful for histological confirmation of successful treatment [14]. Lastly, we cannot control the quality of primary data, and pathological diagnoses from multiple hospitals will lead to inevitable bias.