Introduction
Adenocarcinoma of the esophagogastric junction (AEG) is an adenocarcinoma located at the junction of the esophagus and gastric cardia, and its incidence has increased in recent years [1–3]. Because tumors at this site have special biological characteristics, radical surgery remains the primary treatment option [4]. Siewert type II AEG is defined as an AEG in which the center of the tumor is located 1 cm above the dentate line to 2 cm below the dentate line [5]. Due to the tumor’s special anatomical location, its biological behaviors are complex; the extent of tumor invasion and lymph node metastasis present certain difficulties in selecting the treatment strategy, surgical method and resection range, and clinical surgeons have directed considerable attention to these issues.
In recent years, the continuous progress in laparoscopic techniques and studies of lymph node metastasis in Siewert type II AEG have shown that mediastinal lymph node metastasis of Siewert type II AEG mainly affects the inferior mediastinum; the most commonly affected lymph node is No. 110, with a metastasis rate of 9.0%, while the rates of metastasis to lymph nodes No. 111 and No. 112 lymph nodes are 3.4% and 2.0%, respectively [6]. It has also been found that for Siewert type II AEG with esophageal invasion < 3 cm, transabdominal surgery is superior to transthoracic surgery [7]. Therefore, transabdominal radical surgery for Siewert type II AEG has gradually gained recognition and is increasingly performed.
However, if the extent of esophageal invasion of Siewert type II AEG is greater, the location of the esophageal stump is often high to ensure a negative resection margin, which increases the difficulty of laparoscopic intrathoracic esophagojejunostomy. At present, the most commonly used device-assisted anastomosis methods are overlap anastomosis using a linear stapler and the overlap and transorally inserted anvil (OrVil) anastomosis using a circular stapler [8]. Each anastomosis method has particular advantages and limitations, and the selection of the most suitable anastomosis method requires further study.
Therefore, we report here our experience with the use of overlap and OrVil anastomosis in transabdominal radical surgery for Siewert type II AEG and assess the postoperative surgical outcomes according to the type of anastomosis.
Aim
This retrospective study aimed to compare the efficacy and safety of overlap versus OrVil anastomosis in transabdominal radical surgery for Siewert Type II adenocarcinoma of the esophagogastric junction.
Material and methods
Patients
A total of 34 patients who underwent transabdominal radical surgery and intrathoracic anastomosis for Siewert type II AEG at our center between January 2018 and June 2019 were retrospectively analyzed. The patients had stage II and III Siewert type II AEG. The clinical tumor depth was T2–T3, with no distant metastasis. The preoperative imaging examination did not show clear upper and middle mediastinal lymphadenopathy. A portion of the data from 10 of these patients who underwent OrVil anastomosis was reported in a previous study by the authors [9].
Surgical method
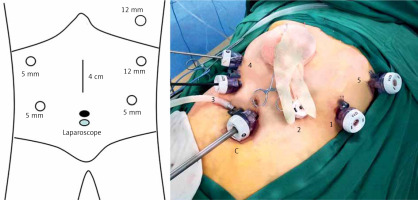
The patient was placed in the lithotomy position, with the head raised to approximately 45° to 60°. The surgeon was on the left side of the patient, the first assistant was on the right side of the patient, and the laparoscopic camera operator was between the patient’s legs. The six-port method (Figure 1) was used: the camera port was located below the umbilicus, Port 1 (12 mm) was located 2 cm below the costal margin on the left anterior axillary line, Port 2 (5 mm) was located 2 cm above the umbilicus on the left midclavicular line, Port 3 (5 mm) was located 2 cm above the umbilicus on the right midclavicular line, and Port 4 (5 mm) was located 2 cm below the costal margin on the right anterior axillary line. Port 5 (12 mm) was located in the sixth and seventh intercostal space on the right anterior axillary line (Figure 1).
All patients underwent radical total gastrectomy (D2 lymph node dissection). After the duodenum was severed, dissection was performed from the right side of the esophagus to the left. The phrenoesophageal ligament was incised. The subcardiac sac was exposed on the right side, and on the left side, an approximately 8-cm incision in the left diaphragm was made, starting from the upper left side of the esophagus. Inferior mediastinal lymph node dissection was performed: the upper boundary of the dissection range was the level of the inferior pulmonary vein, the lower boundary was the foot of the diaphragm, the anterior boundary was the posterior wall of the pericardium, the posterior boundary was the front of the thoracic aorta, and the bilateral boundary was the inferior pulmonary ligament. The lower thoracic paraesophageal lymph node (No. 110), the supradiaphragmatic lymph node (No. 111) and the posterior mediastinal lymph node (No. 112) were completely cleared. Esophageal mesangial denudation was performed starting at the level of the inferior pulmonary veins. We adopted two different intrathoracic anastomosis modalities for the esophagojejunostomy.
An intraluminal linear cutter stapler was inserted at Port 5, and the esophagus was resected at 3 cm above the upper edge of the tumor. The specimen was removed through a midline epigastric incision and sent for intraoperative frozen sectioning, and anastomosis was performed after the upper resection margin became negative.
Anastomosis method: Only laparoscopic OrVil anastomosis or overlap anastomosis was used.
OrVil anastomosis: The jejunum was cut at approximately 30 cm from the distal end of the ligament of Treitz. The antimesenteric border of the small intestine was incised and penetrated at the distal small intestine stump, and the stapler body was placed approximately 30 cm from the distal small intestine stump. The anvil of the OrVil device was placed through the mouth, and fully endoscopic end-to-end anastomosis of the esophagus and jejunum was performed. The anastomosis was sutured in the serosa-muscular layer. The jejunum incision was closed with a linear cutter stapler.
Overlap anastomosis: The jejunum was cut approximately 30 cm from the distal end of the ligament of Treitz. At approximately 60 cm from the distal small intestine stump, the jejunum was incised, and a linear cutter stapler was placed. Fully endoscopic side-to-side anastomosis of the anterior wall of the jejunum and the posterior wall of the esophagus was performed, and the common opening was closed manually with barbed sutures.
The type of complementary reconstruction for the two groups is ROUX-EN-Y.
Ethics statement
This study was approved by the ethics committee of the Sixth Medical Center, PLA General Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of our hospital and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual patients included in the study. Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Results
Table I shows the clinical characteristics of the patients in the two groups: there was no significant difference between the two groups in terms of sex, age, body mass index or length of esophageal invasion. The average tumor size was 7.5 ±2.4 cm in the OrVil group and 4.3 ±1.9 cm in the overlap group (p < 0.05). Table II shows the relevant surgical data for the two groups of patients. In terms of duration of surgery, anastomosis time and total number of lymph nodes dissected, there was no statistically significant difference between the two groups. In terms of the distance from the upper esophageal resection margin to the tumor, the OrVil group (3.2 ±0.84 cm) had a longer distance than the overlap group (2.4 ±0.6 cm). No cancer was found in the upper and lower incisal margin of the patients in the two groups by intraoperative freezing and postoperative pathology. Table III shows the postoperative complications data for the two groups. The OrVil group had no complications such as anastomotic leakage or stenosis, while the overlap group had two cases of anastomotic leakage accompanied by anastomotic stenosis and one case of postoperative duodenal stump leakage that caused postoperative bleeding. The patients with anastomotic leakage were cured after conservative treatment, and the subsequent anastomotic stricture was completely relieved by endoscopic balloon dilatation. The patients with duodenal stump leakage underwent the second operation to stop the bleeding, followed by abdominal irrigation and drainage, and were cured and discharged 1 month after operation. The main postoperative complications in the two groups of patients were pulmonary infection and pleural effusion. The OrVil group had one case of pulmonary infection and two cases of pleural effusion, while the overlap group had four cases of pulmonary infection and 18 cases of pleural effusion. All patients with pleural effusion were treated with ultrasound puncture and drainage, and all patients were cured by conservative treatment. Although the incidence of complications was lower in the OrVil group, this could be explained by the smaller number of patients in this group; therefore, further observation and evaluation are required in our subsequent work.
Table I
Clinical characteristics of the patients
Table II
Surgery and anastomosis
Discussion
Regarding total laparoscopic surgery for Siewert type II AEG, one of the difficulties in intrathoracic esophagojejunostomy is the narrow mediastinal space and difficult transabdominal operation. The advantage of thoracic surgery lies in the large surgical space for thoracoscopic intrathoracic digestive tract reconstruction. However, the thoracoabdominal surgery methods described by Ivor-Lewis and McKeown [10, 11] require an intraoperative change of the patient’s position and prolonged operation times. Furthermore, it is difficult to determine the condition of the abdominal cavity after the patient’s position is changed, and serious problems such as mesenteric torsion and bleeding tend to occur. Therefore, if complete laparoscopic esophagojejunostomy is performed under direct vision, a better and satisfactory operation space and visual field can be obtained [12]. At our center, we found that after incising the left diaphragm and entering the thoracic cavity, the surgical space was significantly increased compared with the transesophageal hiatal approach, which greatly reduced the duration of intrathoracic esophagojejunostomy. The angle was even better when esophageal transection was performed through the auxiliary port in the left intercostal space, and a more satisfactory surgical margin could be obtained. Moreover, both overlap anastomosis and OrVil anastomosis could be completed under direct vision, with a large operating space and satisfactory anastomosis results.
The use of circular staplers for esophagojejunostomy is familiar to most gastrointestinal surgeons. However, the placement of the anvil of a circular stapler under laparoscopy is a difficult problem that plagues surgeons. It has been reported that the placement of the anvil of a circular stapler requires manual purse-string suturing after the esophagus is transected and the placement of the anvil by reverse-puncture method after the esophagus is incised. The posterior mediastinum has a relatively narrow space, the placement of the anvil by conventional manual purse-string suture is time-consuming and laborious, and there are many associated complications, such as postoperative anastomotic leakage, anastomotic stenosis and infection [13]. Therefore, placement of the anvil with conventional manual purse-string suture is rarely used in clinical practice at present. In 2009, Omori et al. [14] reported a method for placing the anvil of a circular stapler that they called the reverse puncture method. Although this method is relatively easy to perform, it may increase the chance of tumor shedding and implantation during esophageal incision. Furthermore, it is difficult for a linear cutter sealer to transect the esophagus in the thoracic cavity if the esophageal stump is high. Although it has been reported that 3D laparoscopic hand-suture esophagojejunostomy is safe and repeatable, the median suture time is 55 min, and the technique is difficult [15]. In contrast, for OrVil anastomosis, the anvil is placed through the mouth, and there is no need for purse-string suturing of the esophageal stump, which greatly simplifies the placement process [16]. Jeong and Park [17] first reported this anastomosis method in 2009. Earlier studies suggested that OrVil anastomosis has the disadvantages of requiring the intraoperative involvement of an anesthesiologist and a high degree of surgical difficulty. Furthermore, there is a possibility of complications such as esophageal mucosal injury and abdominal infection, caused by the placement of the anvil through the mouth [18–20]. However, we have overcome these challenges by improving on the original procedure. First, the anvil was placed by a physician while the patient was positioned with the neck retroverted and the anesthesiologist supporting the mandible, and placement was completed in all 10 patients without the removal of the tracheal tube balloon. Before the completion of the anastomosis, we inserted the laparoscope through the left chest auxiliary port and observed the connection between the anvil and the stapler body in the thoracic cavity; the field of vision was significantly better than from the transabdominal angle, and the anastomosis was precise. While the abnormal drainage of the abdominal cavity reported in previous studies may be related to poor anastomotic healing and anastomotic leakage, none of our 10 patients who underwent OrVil anastomosis developed abdominal infection. Finally, because we performed end-to-end esophagojejunostomy, the OrVil anastomosis provided a larger surgical margin with less local tissue tension. For Siewert type II AEG with a stage ≥ cT2, a retrospective study [21] showed that among 45 cases with positive margins, 91% had a resection margin distance < 3 cm, which is a risk factor for positive margins. Another retrospective study that enrolled 505 AEG patients [22] found that an ex vivo esophageal resection margin distance > 3.8 cm (an in vivo distance of approximately 5 cm) was an independent risk factor for a poor prognosis. Our study found that OrVil anastomosis can provide an upper resection margin distance of 3.2 ±0.84 cm. Therefore, for Siewert type II AEG with a stage ≥ cT2 and esophageal invasion > 2 cm, we believe that the use of OrVil anastomosis can achieve safer and more satisfactory esophageal resection margins.
The Japanese researchers Inaba et al. [23] first reported the use of esophagojejunal overlap anastomosis for digestive tract reconstruction after laparoscopic total gastrectomy in 2010. This procedure has the advantages of antegrade peristaltic emptying of the anastomosis, a wide anastomotic caliber and low anastomotic tension. Since then, there have been some minor improvements in this anastomosis method, mainly focusing on the selection of anastomotic stoma and the suturing of the common opening. At our center, we have also performed side-to-side anastomosis between the posterior wall of the esophagus and the anterior wall of the small intestine after barbed suturing on both sides of the esophageal stump and pulling down the esophagus. The common opening was closed by manual continuous suture with a fixed barbed suture. The average anastomosis time was 24.40 ±6.1 min, slightly less than that of OrVil anastomosis. The p-value was > 0.05, which may be related to the small sample size. However, the length of the small intestine and esophagus required for overlap side-to-side anastomosis is significantly longer than that required for end-to-end anastomosis with a circular stapler. The average distance from the upper esophageal resection margin to the tumor in the 24 overlap anastomoses performed at our center was 2.4 ±0.6 cm, which is shorter than that of the OrVil anastomosis. Therefore, for Siewert type II AEG with short mesentery and esophageal invasion > 2 cm, overlap anastomosis should be selected with care.
According to the comparison of postoperative complications between the two anastomosis methods, a multicenter study of 272 patients showed that the OrVil group was associated with less intraoperative hemorrhage (p < 0.001) and a lower postoperative anastomotic leakage rate (p = 0.033). Multivariate logistic regression analysis showed that anastomotic technique and pulmonary infection were independent factors for the development of postoperative anastomotic leakage (p < 0.05) [24]. Pulmonary infection after intrathoracic esophagojejunostomy is more common. Hong et al. reported that the incidence of pulmonary complications after OrVil was about 14.5% [25]. Kan et al. reported that 39 (18.14%) patients had postoperative pulmonary complications after OrVil anastomosis [26]. The incidence of postoperative pleural effusion in the two groups of patients was 0.2 (2/10) for the OrVil group and 0.75 (18/24) for the overlap group. Although p < 0.01 for the two groups, there was no significant difference in pulmonary infection or anastomotic leakage. This finding may be due to the fact that closed thoracic drainage was not routinely placed in the overlap group rather than to the difference in the anastomosis methods.
The limitations of this study are the relatively small number of patients enrolled and the lack of observation of long-term effects after surgery. Because the coronavirus disease 2019 (COVID-19) outbreak affected patient enrollment and postoperative follow-up efforts, the retrospective analysis included only patients treated up to June 2019. Long-term efficacy and other aspects require further consideration in future work.
Conclusions
Due to the special tumor location of Siewert type II AEG, laparoscopic total esophagojejunostomy and its difficulty are enduring focuses of discussion, and there is no clear conclusion regarding the choice of circular stapler or linear stapler for anastomosis. Based on our work, we believe that for Siewert type II AEG patients with a stage ≥ cT3, an esophageal invasion length > 2 cm and a short mesentery, OrVil anastomosis should be performed to achieve a more satisfactory esophageal resection margin. In contrast, for patients with Siewert type II AEG with esophageal invasion < 2 cm, overlap anastomosis is more convenient. There was no significant difference in postoperative complications between the two methods.