Introduction
Arboviruses-related infections represent emerging viral diseases worldwide, particularly in tropical areas. They are transmitted to humans primarily through bites of mosquitoes, especially of the species Aedes aegypti and to a lesser extent Aedes albopictus, ticks, sand flies and biting midges [1]. Different types of infections include dengue fever (DF), yellow fever, chikungunya, Zika, Japanese encephalitis, and Rift Valley fever. Dengue fever, the most common infection, is caused by the dengue virus (DENV), a flavivirus and member of the Flaviviridae family which exists under four distinct but closely related serotypes: DENV-1, DENV-2, DENV-3 and DENV-4. It is estimated that 390 million people worldwide are at risk of catching dengue infection annually, with approximately 20,000 deaths. Of these 390 million cases, 96 million (25%) show clinical symptoms [2]. In Africa, the epidemiology of dengue is unclear and sporadic cases of dengue infection have been reported in about 24 countries [3]. In Cameroon, there is no exact figure for how many people live with DF but studies have reported infection cases in Douala and Yaoundé [4-6]. While previous studies have shown that dengue can lead to acute liver diseases [7-9], some reports indicate that coinfection cases with viral hepatitis may occur, leading to more severe complications. In Guangzhou city (China), for instance, of the 353 patients who tested positive for dengue, 8% had chronic dengue-hepatitis B virus (HBV) co-infection with aberrant cytokine secretion [10]. However, nothing was said about the clinical consequences. Later, Argawal et al. (2011) found fulminant hepatitis in an HBV-DENV coinfected carrier in India [11].
Hepatitis B is a serious viral disease that attacks the liver, causing both acute and chronic liver diseases (WHO, 2020). It is caused by hepatitis B virus (HBV), a DNA virus belonging to the Hepadnaviridae family. HBV is transmitted through sexual intercourse, exchange of saliva with an infected person, blood transfusion and during delivery [12]. HBV infection is a leading cause of liver cancer and cirrhosis (a chronic liver disease), resulting in disabilities and deaths. According to a recent report from the World Health Organization (WHO), about 296 million people had chronic HBV infection worldwide in 2019, with 820 000 deaths and 1.5 million new infections occurring yearly [13]. In Africa, hepatitis B is estimated to affect 60 million individuals, with at least 200,000 deaths per year [14]. In Cameroon, 12,000 new cases of HBV were reported in 2019, compared to 9,600 in 2018 [15]. According to the same author, Cameroon’s Ministry of Public Health estimates revealed that 13% of the whole population lives with hepatitis B in the country, with about 10,000 deaths annually. A systematic review and meta-analysis conducted by Bigna et al. (2017) reported 11.2% as a pooled seroprevalence of HBV in Cameroon [16]. Another study demonstrated that HBV-infected patients are likely to develop hepatocellular carcinoma below the age of 40 [17]. An epidemiological survey carried out on a young male population (18-23 years old) highlighted variable rates per region ranging from 22.82% in the Far North, 21.53% in the North, and 12.75% in Adamawa, among others, for a general prevalence of 13.01% [18]. These previous findings pointed out that the Adamawa region is one of the areas most affected by hepatitis B. Presently, there is no information on the circulation of this virus in Ngaoundere, especially HBV-dengue coinfection, which also causes liver disease. Furthermore, it is not well known how this co-infection might affect the biochemical parameters of patients. In this study, we examined the frequency of DENV-hepatitis B coinfection and established the correlation with parameters of liver function in people inhabiting Ngaoundere city (Cameroon).
Material and methods
A cross-sectional study was carried out from 25 September to 15 December 2021 on participants consulting at the Ngaoundere Regional Hospital, Adamawa, Cameroon. Ethical authorization was obtained from the hospital ethics review board under the reference number 316/AR/RA/DRSP/HR/NGD. The study included febrile patients aged 15-55 years presenting at least two of the following symptoms: fever, headache, influenza, joint pain, abdominal pain, retro-orbital pain, bleeding, nausea, vomiting, convulsion. Patients vaccinated against hepatitis B or those with chronic liver disease were excluded from the study. Patients having tested positive for viral hepatitis more than 6 months ago, and those who stated that they suffered from cirrhosis, fibrosis, steatosis, and liver failure before the study were considered as patients with a chronic liver disease. The minimum sample size (174 subjects) to be used for this study was calculated by the Lorentz formula using the current HBV prevalence (12.75%) in the Adamawa region [19] with a 0.05 degree of precision and 95% confidence level. For more precision, a higher sample size of 225 was considered.
Participants were first administered a structured questionnaire (supplementary file) that contained demographics, and data in connection to prior exposure to liver disease or dengue infection. After giving their informed consent, the questionnaire was filled out for each patient and 5 ml of his/her blood was sampled in dry tubes. Serum was prepared and kept at –20°C for lipid profile and liver function parameters analysis. Dengue infection was diagnosed using the NS1/IgM/IgG combined kit from Medsinglong Co. Ltd (Guangzhou, China) and HBV was detected using the HBsAg rapid diagnostic test (One-step strip) provided by Swecare diagnostics. According to the manufacturer, the relative reactivity of this test is > 99.0%, and relative specificity > 97.0%, with an accuracy of 98.5%.
Alanine aminotransferase (ALT), γ-glutamyltransferase (γ-GT), and total and direct bilirubin were measured using kits provided by SGM Italia, Roma (Italy). Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein (LDL-C) and triglyceride (TG) were quantified using a lipid analyser (LipiDiag) provided by Medsinglong Global Group Co., Ltd and following the manufacturer’s instructions. This analyser which applies the principle of photochemistry was used with a blood lipid test strip (MSL-301). Briefly, the whole blood sample to be tested was added into the sample area of the strip. In the process of rapid infiltration, blood cells were filtered out or dissolved. The substrate, consisting mainly of plastic shell, PET, glass fibre membrane and reaction membrane contained in serum, is expected to react with enzymes and chemicals such as cholesterol esterase, cholesterol oxidase, lipoprotein lipase, glycerol kinase, glycerol phosphate oxidase, horseradish peroxidase, ascorbic acid oxidase, 4-amino-antipyrin, MAOS and dextran sulfate in the reaction layer, leading to release of a coloured product whose intensity is proportional to the concentration of the cholesterol molecules. The analyser tests the colour intensity of the reaction endpoint at the wavelength of 620 nm, and the substance concentration was calculated by the reflection coefficient. With the following formula the concentration of LDL-C is calculated, and the ratio of total cholesterol TC and HDL-C can also be calculated:
LDL-C = TC – (HDL-C) – TG/5 (mg/dl)
Statistical analysis
GraphPad Prism 5.0.3 (San Diego, USA) was used to analyse the data. To investigate for connections between dengue, viral hepatitis and socio-demographic characteristics in the study population, Fisher’s test was performed. Mean TC, HDL-C, LDL-C, TG, γ-GT, total bilirubin, direct bilirubin, and ALT levels were compared between groups using the non-parametric Kruskal-Wallis test followed by Duncan’s multiple comparison test. The probability level was set to 5% (p < 0.05).
Results
Seroprevalence of HBV and DF infection in the study population
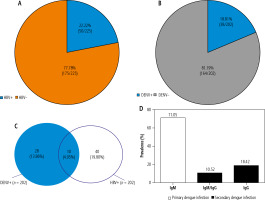
The prevalence of HBV and dengue infection in the study population is presented in Figure 1. Of the 225 subjects investigated, 22.22% (50) were found seropositive to HBsAg (Fig. 1A), and only 202 were screened for dengue infection, with a prevalence rate of 18.81% (38/202) (Fig. 1B). The 23 remaining patients were not analysed due to a lack of test kits. A dual HBV/dengue infection was recorded with a percentage of 4.95% (10/202) (Fig. 1C). Most of the dengue patients had a primary dengue infection (Fig. 1D) as indicated by anti-DENV IgM positive samples (71.05%), compared to 28.94% for secondary dengue infection (10.52% IgM/IgG; 18.42% IgG only).
Socio-professional activities associated with HBV infection in the study population
The statistical association between HBV seropositivity and socio-demographic factors is presented in Table 1. As shown by this table, age, place of residence and gender did not significantly affect the HBV seropositivity (p > 0.05). However, some groups tend to be more susceptible to the infection, although this exposure is not significant. For example, people between 25 and 35 years old showed a higher relative risk (RR) compared to those aged between 45 and 55 years. Interestingly, a significant association was observed with socio-professional activities. Indeed, the informal sector exhibited a significantly higher RR compared to households (RR = 2.23 vs. 1.00, p < 0.05).
Table 1
Statistical association between hepatitis B virus (HBV) status and socio-demographic factors
Socio-demographic factors associated with DF infection
After checking the association between socio-demographic factors and HBV status, we also conducted the same analysis on DENV status (Table 2). In general, no statistical association was found between age, gender, socio-professional activities, place of residence and DENV seropositivity. However, some groups within the factors analysed tend to be more vulnerable to dengue infection due to their high RR (RR > 2).
Table 2
Statistical association between Dengue status and socio-demographic factors
Influence of monoinfected and coinfected subjects on liver injury
Previous studies have demonstrated that HBV and DENV are each able to cause liver disease. Considering this fact, it was important to check whether the co-infection impairs liver functions. As shown in Table 3, co-infected subjects were not at a significantly higher risk of liver injury than mono-infected subjects. They shared the same RR for total bilirubin (RR = 1.05, 70% vs. 66.66%) and direct bilirubin (RR = 1.05, 80% vs. 75.75%) compared to the HBV mono-infected group. However, for γ-GT (RR = 1.74, 90% vs. 51.51%) and ALT (RR = 1.52, 60% vs. 39.39%), they tended to be more exposed to abnormal values although the RRs were not statistically significant.
Table 3
Statistical association between parameters of liver function and type of infection
Influence of coinfection and mono-infection on ALT, γ-GT, LDL-C, and TG levels
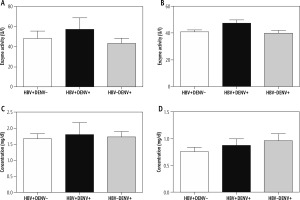
The mean values of liver injury parameters are shown in Figure 2. No significant difference was found between coinfected and monoinfected subjects regarding ALT, γ-GT, direct and total bilirubin levels. How-ever, a mild increase of ALT (57.10 ±11.62 vs. 43.43 ±5.08 IU/l) and γ-GT (47.2 ±2.5 vs. 39.75 ±2.18 IU/l) was noted compared to the dengue-monoinfected group.
Fig. 2
Mean values of liver function parameters of the coinfected subjects in comparison with HBV and DENV monoinfected subjects. A) ALT values, B) γ-GT, C) total bilirubin, D) direct bilirubin
According to the reference values used at the Laboratory of the Ngaoundere Regional Hospital, the normal values of the lipid parameters included TC < 200 mg/dl, 35 < TG < 160 mg/dl, HDL-C > 35 mg/dl, and LDL-C < 130 mg/dl. All the values displayed in Table 4 are within the normal range. However, for LDL-C, and TG, a small increase was observed in the coinfected group in comparison to the monoinfected groups, although not significant. Similar findings were recorded for the TC/HDL-C ratio.
Table 4
Mean values of lipid parameters of the coinfected subjects in comparison with HBV and DENV monoinfected subjects
Discussion
This research was carried out to determine the prevalence of HBV-DF co-infection at the Ngaoundere Regional Hospital, and the effects of co-infection on biochemical parameters linked to liver function. The results indicated a general prevalence rate of 22.22% (50/225) for hepatitis B in the study population, while prevalence rates of 18.81% (38/202) and 4.95% (10/202) were obtained for dengue virus and HBV-DENV coinfection respectively. HBV prevalence was higher than that earlier reported in the Adamawa region (12.75%) [18]. This result is an indication that Adamawa, and more specifically Ngaoundere, remains a highly endemic region for HBV infection since inhabitants of the region largely minimize barrier measures. Indeed, recent reports showed sex tourism on the rise in that town, increasing the circulation of sexually transmitted diseases [20]. Of the 38 patients tested positive for dengue, 71.05% had a primary dengue infection due to a higher proportion of anti-DENV IgM antibodies. The very low proportion of anti-DENV IgG obtained suggests that DENV transmission is seasonal in Ngaoundere, and the seemingly high IgM to IgG ratio could be because the infection was still recent at the time of blood collection from the patients. These data also confirm that dengue is endemic in the Adamawa region like malaria, as previously reported by Galani et al. (2021), who detected a prevalence rate of 8.95% in malaria patients in Ngaoundere [21]. Moreover, most dengue-infected people had primary dengue infection. This is consistent with previous studies that reported arbovirus vectors in different localities of Ngaoundere from September to November [22]. Ngaoundere, therefore, seems to be a place highly exposed to the circulation of arboviral infections.
To the best of our knowledge, there is no information on the presence of dengue in HBV patients in Cameroon or elsewhere in Africa. The coinfection rate found in this study is lower than that reported in Guangzhou, China (8%) [10]. The discrepancy with the Chinese study could be due to our small sample size. However, this is clear evidence that co-infection between arboviruses and viral hepatitis B is possible. To explain these data, the association between prevalence rates and socio-demographic factors was analysed. However, due to the low frequency of coinfected individuals, it was more useful to concentrate on the association between separate infections and socio-demographic factors. The above-mentioned Chinese study showed that ALT levels were higher in patients with either HBV or dengue infection and surprisingly coinfected patients made less interleukin (IL)-6 and tumor necrosis factor α (TNF-α) than patients with only dengue infection but had similar levels of IL-4, IL-10, and interferon γ (IFN-γ) in comparison to those with only dengue infection. Thus, HBV co-infection seems to alter the cytokine production pattern when patients contract dengue infection. This implies that co-infection increases the chances of liver dysfunction, thereby increasing the severity of infection.
There was no statistically significant association between HBV seropositivity and socio-demographic factors (age, place of residence and gender) (p > 0.05). However, due to their high RR, some age groups (25-35 years) tend to be more susceptible to the infection than those of 45-55 years, even though this exposure was not significant. Indeed, a retrospective study in China showed that HBV infection increases with age, with an adjusted odds ratio of 11.61 for people more than 21 years old. Similarly, a study conducted in Dschang revealed that age, level of education and occupation are all associated with hepatitis B [19]. People in the 17-31 age range were found to be more exposed to HBV, with a prevalence rate of 12.64%. Unlike, gender, age, and place of residence, a significant association was observed within the socio-professional activities. For instance, the informal sector showed a significantly higher RR for hepatitis B (p < 0.05) compared to households. A study conducted in Burkina Faso showed that people working in the informal sector were among the groups most affected by hepatitis B, with a prevalence rate of 15.95% [26]. Also, a study carried out in Pakistan indicated that the strong spread of hepatitis B in the private and informal sectors could be due to poverty, illiteracy, and multiple social and cultural barriers prevailing at any given time in a country [27]. According to the same study, private and informal sector workers hardly earned enough to go in for vaccination and quality medical services; as a result, they tend to go for cheaper treatment and general practitioners in search of treatment for diseases including hepatitis B.
As far as dengue is concerned, no significant association was also found between age, gender, socio-professional activities, place of residence and DENV seropositivity. However, some groups within the factors analysed tend to be more vulnerable to dengue infection due to their high RR, especially people from Ngaoundere 1 (RR = 2.81), Ngaoundere 2 (RR = 1.83), households (RR = 2.55), private sectors (RR = 2.47), and people aged > 25 years old (RR > 1.6). These factors seem to play a role in the endemic character of dengue in the Adamawa region. Previous studies proved that dengue seroprevalence increased rapidly with age, reaching 97% by 60 years [28]. Also, some neighbourhoods such as Onaref, Mardock, Balaji I and Balaji II, which all belong to Ngaoundere 2 and 1, were found to host important areas for the circulation of arbovirus vectors [22].
A study conducted in Faisalabad, Pakistan showed that socioeconomic disparities in the community make some groups more vulnerable to dengue infection [23]. According to this report, 22.9% of the surveyed participants (4.8% positive for IgM and 18.1% positive for IgG) were dengue positive. Moreover, the highest (45%) seroprevalence was reported in the most socio-economically vulnerable lower class, indicating that socio-economic factors play a significant role in the prevalence of dengue. Based on the new classification of WHO of 2009 [24], dengue fever may be grouped into two categories, namely non-severe dengue (NSD) and severe dengue fever (SDF). NSD itself also may be split into two categories: NSD with no warning signs (NSD-W) and NSD with a warning sign (NSD+W). One criterion to differentiate SDF from other types of dengue is the relatively high aspartate aminotransferase (AST) or ALT value (> 1000 IU/l). In this study, none of the DF patients was found with an ALT value reaching this minimum threshold. All of them are therefore considered as NSD patients. Unfortunately, as previously reported [25], ALT values could not discriminate NSD+W from NSD-W patients due to overlapping.
The impact of dengue-viral hepatitis coinfection on liver function has been sparsely evaluated in the literature. In this study, we examined this effect by carrying out a logistical regression analysis. As shown in Table 3, co-infected patients were not found to be at a significantly higher risk of liver injury relative to mono-infected subjects. However, they have higher mean ALT and γ-GT values compared to other groups, although the differences are not significant. Indeed, mean ALT and γ-GT values, although higher in the coinfected group, did not differ significantly from those of dengue-monoinfected patients. This finding emphasizes the need to look at dengue as a probable cause of severe liver failure in hepatitis B patients. A Vietnamese study found that chronically HBV-dengue coinfected patients had significantly higher ALT and AST levels than monoinfected subjects during the critical period when they develop clinical signs of DF [29]. However, this effect was minor and there was no association between these parameters during the convalescent period. Therefore, the lack of statistical association found in the current study could be linked to the fact that patients were not in the critical dengue phase.
As for lipid parameters, no significant changes were noted compared to the reference values and between coinfected and monoinfected groups. However, a small increase in LDL-C, TG levels and the TC/HDL ratio was found in the coinfected group, suggesting thereby that concurrent HBV/DENV infection might likely interfere more with lipid metabolism. Indeed, HBV and DENV are both enveloped viruses known to use lipids as key elements in their replication process. It has been shown for example that DENV may alter the fatty acid synthesis by direct binding to fatty acid synthase, thereby favouring the re-localization of this enzyme in the replication complex [30]. Therefore, fatty acid accumulates in the intracellular compartment, leading to increased levels of triglycerides. This could partly explain the mild increase of TG observed in this study. Earlier experimental studies also revealed that HBV infection could both increase lipogenesis and fatty acid synthesis and stimulate lipolysis [31], which remains somewhat controversial. Recent evidence demonstrated that serum lipids may serve as predictors of dengue severity with lower TC and LDL-C values [32, 33] or biomarkers of liver alteration with significantly lower TC, LDL-C, and HDL-C levels and lower liver steatosis [34] in comparison to controls. The results obtained from this study are different from these previous findings. However, some authors reported that HBV/HCV coinfected patients had significantly higher TG levels compared to the HBV-monoinfected group [35], as found in the present study. Further investigations are therefore necessary to confirm these observations.
Despite the efforts undertaken to detect HBV-dengue coinfection in the sampled patients, this study did not assess the DENV serotypes, the HBV genotypes, markers of HBV chronic infection, and the viral loads of both mono- and coinfected patients. This could have possibly permitted us to better characterize their clinical profiles and therefore judge their impact and the course of the disease. We must admit that more biochemical parameters could have been evaluated as well as the sample size. The obtained results give an epidemiological basis for more important clinical and experimental research.
Conclusions
In conclusion, HBV and DENV infection are strongly present in the Cameroonian population sampled in Ngaoundere city, with a coinfection rate evaluated at 4.95%. Coinfected patients showed a higher relative risk of liver injury compared to monoinfected patients although not significant. Their higher mean TC, TG, and LDL-C suggest that they might be more affected by dyslipidaemia and coronary heart disease. More investigations regarding their effect on biochemical parameters are required to understand their clinical relevance.