Introduction
Vitiligo is an immune-mediated inflammatory skin disease, characterized by depigmented patches resulting from the loss of functional melanocytes [1]. The pathogenesis of vitiligo remains enigmatic, involving a complex interplay of biochemical, environmental, and immunological factors within a genetic background [2]. The autoimmune hypothesis is particularly compelling as multiple studies have observed that activation of the innate immune response and subsequently alterations in CD4+ T cell function, lead to the activation of autoreactive melanocyte-specific cytotoxic T cells in vitiligo pathogenesis [3, 4].
Accumulating evidence has revealed the alteration in the specialized subpopulation of CD4+ T cells, including T helper 1 (Th1), Th2, Th17, and regulatory T cells (Tregs) have been implicated in vitiligo [5]. Specifically, the immune response of vitiligo is skewed towards Th1 or Th17 and away from Tregs responses [6]. Cytokines play a crucial role in facilitating cellular communication and networking [7]. The secretion of multiple cytokines by these T cells can result in melanocyte destruction, either through direct mechanisms or by activating autoreactive T cells in vitiligo [8–10]. Furthermore, chemo-attractant cytokines play a pivotal role in recruiting leukocytes to inflammatory sites, amplifying the inflammatory reaction, and inducing melanocyte death [11]. Serum cytokine levels can be measured for monitoring disease severity and treatment efficacy in many studies [12–14].
To date, the unclear pathogenesis of vitiligo has posed challenges for effective clinical treatment. The recommended treatment options for vitiligo include topical and systemic glucocorticoids, topical calcineurin inhibitors, phototherapies, and other immunosuppressants [15]. Systemic glucocorticoid treatment offers one of the most effective treatment modalities for active vitiligo, but the efficacy still varies across patients and treatment resistance is often encountered [16]. Therefore, predicting treatment efficacy carries significant implications for selecting suitable treatments for vitiligo patients individually.
Compound betamethasone injection (CBI) contains quick-acting betamethasone sodium phosphate and long-acting betamethasone dipropionate [17]. For patients with active vitiligo, CBI presents an attractive therapeutic option due to its convenient application and ability to provide long-term disease control [18]. Therefore, it is meaningful to identify indicators to predict treatment effects.
Aim
The aim of the study was to assess the serum levels of pro-inflammatory cytokines of innate immunity (TNF-α, IL-1β, IL-6), Th1 (IFN-γ, IL-2), Th17 (IL-17), Treg (IL-10) cytokines, and chemokines (IL-8, CXCL10) pre- and post-CBI treatment in patients with active vitiligo. In addition to monthly CBI treatment, vitiligo patients also received topical halometasone cream for body lesions and 0.1% tacrolimus ointment for facial lesions twice daily. Our findings may provide a novel insight into vitiligo pathogenesis and personalized treatment, especially for new treatment strategies in patients resistant to glucocorticoid therapy.
Material and methods
Participants
Thirty-one consecutive consenting patients with active vitiligo were included. Clinical diagnosis of vitiligo and the stages of disease activity were determined by three dermatologists based on the consensus on the diagnosis and treatment of vitiligo in China [19]. The inclusion criteria consisted of patients over 18 years old and patients who exhibited new spreading lesions or the enlargement of previous lesions within the past 3 months. Exclusion criteria included pregnancy, receiving any steroids or immunosuppressive treatment within 12 weeks. Clinical examination was carried out to determine the affected BSA. Disease duration was defined as the time between vitiligo onset and testing. The control group comprised 20 age- and gender-matched healthy volunteers without any personal or family history of autoimmune disease. All participants provided written informed consent to study enrolment and data analysis.
Intervention
Diprospan is a compound betamethasone solution (Shanghai Schering-Plough Pharmaceutical, Shanghai, China). Vitiligo patients were treated using 1 ml intramuscular injections of diprospan (containing 2 mg betamethasone sodium phosphate and 5 mg betamethasone dipropionate) once per month, and the total treatment period was 3 months. All patients received topical halometasone cream (Hong Kong Bright Future Pharmaceuticals, China) for body lesions and topical 0.1% tacrolimus ointment (Astellas Toyama Company Limited, China) was applied for facial lesions twice daily. None of the patients had received phototherapy. According to the response to therapy, we classified patients into two groups: responder group (patients with stable disease or got repigmentation) and non-responder group (patients with progressive disease).
Cytokine analysis
Peripheral blood samples of 5 ml were collected using a serum-separating tube, allowed to clot undisturbed at room temperature for 20–30 min, and then centrifuged at 3,000 rpm for 10 min. The resulting supernatant was aspirated into a fresh polypropylene tube and stored at –80°C for subsequent unified testing. The serum cytokine levels were assessed in 31 vitiligo patients before and after treatment, as well as in 20 healthy controls. Serum cytokine levels of TNF-α, IL-1β, IL-6, IFN-γ, IL-2, IL-17, IL-10, IL-8, and CXCL10 were measured by sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, China). The ELISA tests were performed according to the manufacturer’s instructions. The resultant absorbance was measured using a spectrophotometric microplate reader (Thermo Fisher Scientific, USA).
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Xijing Hospital.
Statistical analysis
Statistical analysis was conducted using SPSS Version 26 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 software (GraphPad, La Jolla, CA, USA). For normally distributed continuous variables, group differences were assessed using one-way analysis of variance (ANOVA), followed by Bonferroni’s post-hoc test. For non-normally distributed data, the Kruskal-Wallis test was employed. χ2 test was used to test for differences between categorical variables. Spearman’s correlation coefficients were used to quantify the correlations between cytokines and clinical manifestations. P-value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
Thirty-one patients with active vitiligo were recruited, including 14 (45.2%) males and 17 (54.8%) females. The mean age of patients was 35.2 ±11.3 years. The duration of disease ranged from 4 months to 43 years with a mean of 9.3 years. The body surface area (BSA) involved ranged from 1% to 11.2% with a mean of 4.3%. Three (7.7%) patients had other autoimmune diseases, and 9 (29.0%) patients had a family history of vitiligo or other autoimmune diseases. There were no significant differences in age and gender among the two subgroups of vitiligo patients and controls (p > 0.05). Demographic and clinical features of patients are reported in Table 1.
Table 1
Clinical characteristics of patients with vitiligo
Cytokine levels at baseline before CBI treatment
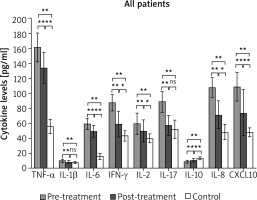
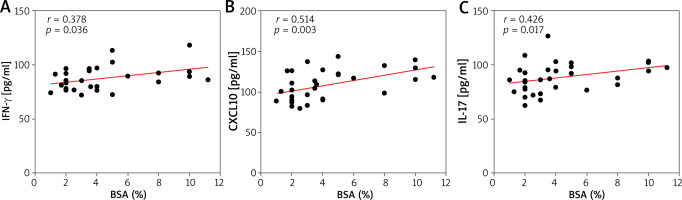
Before CBI treatment, vitiligo patients exhibited elevated serum levels of TNF-α, IL-1β, IL-6, IFN-γ, IL-2, IL-7, IL-8, and CXCL10 compared to the control group, while the level of IL-10 was lower (Figure 1). Additionally, we noted a significant positive correlation between BSA and serum levels of IFN-γ (r = 0.378, p = 0.036), IL-17 (r = 0.426, p = 0.017), and CXCL10 (r = 0.514, p = 0.003) (Figures 2 A–C).
Cytokine levels of vitiligo patients various after CBI treatment
After 3-month courses of CBI treatment, there was a marked decrease in serum TNF-α, IL-1β, IL-6, IFN-γ, IL-2, IL-17, IL-8, CXCL10 and an increase in IL-10 in all vitiligo patients as a group. Among them, the levels of IL-1β and IL-17 were restored to a level comparable to that of the control group (Figure 1).
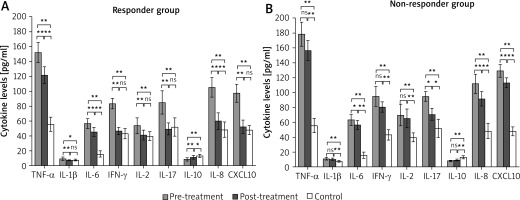
Subsequently, vitiligo patients were categorized into responder and non-responder groups based on their response to CBI treatment. Intriguingly, we observed significant differences in cytokine level changes between the two groups. In the responder group, there was a significant amelioration in the levels of multiple cytokines including TNF-α, IL-1β, IL-6, IFN-γ, IL-2, IL-17, IL-10, IL-8, and CXCL10. Moreover, the serum levels of IL-1β, IFN-γ, IL-2, IL-17, and CXCL10 were even restored to a level similar to that of controls (Figure 3 A). By contrast, although IL-6, IL-17, IL-8, and CXCL10 decreased after treatment in the non-responder group, the serum levels of TNF-α, IL-1β, IFN-γ, IL-2, and IL-10 did not exhibit significant differences between pre- and post-treatment levels (Figure 3 B).
Figure 3
Serum cytokine profiles of patients with active vitiligo pre- and post-treatment. Patients were divided into responder (A) and non-responder (B) groups according to response to therapy. Data were displayed as mean ± standard deviation. P-values were calculated using one-way analysis of variance or Kruskal-Wallis test. *P < 0.05; **p <0.01
Discussion
In this study, we detected changes in cytokine levels in active vitiligo patients before and after CBI treatment. Our findings suggested that the resistance to CBI treatment in vitiligo patients may be attributed to multiple cytokine-mediated immune abnormalities. By analysing the differences in serum cytokines between the responder and non-responder groups, we identified certain cytokines that may be crucial for evaluating or predicting CBI resistance in vitiligo patients.
Vitiligo is a depigmenting and inflammatory disease mediated by T lymphocytes. Immune responses involving cytokines and CD4+ T cell subsets are believed to contribute to the pathogenesis of vitiligo [20]. At present, controlling the progression of vitiligo poses a significant challenge. Glucocorticoids exhibit a broad spectrum of anti-inflammatory and immunosuppressive effects, effectively suppressing cytokine release and mitigating immune-mediated damage [21]. CBI, a long-acting and safe glucocorticoid, has demonstrated efficacy in the treatment of vitiligo through multiple studies [22, 23]. However, the immunomodulatory mechanism underlying CBI therapeutic effects remains to be elucidated. Our study provided evidence supporting the broad immunomodulatory role of CBI in treating active vitiligo.
In this study, the levels of pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6, were significantly elevated in patients with vitiligo. Generally, IL-6, TNF-α, and IL-1β can facilitate the differentiation of Th1 and Th17, subsequently promoting the immune response mediated by these cell subsets and leading to immune dysregulation in vitiligo [24, 25]. TNF-α promotes the secretion of IFN-γ by promoting the differentiation of cytotoxic T lymphocytes [26]. At the same time, through various mechanisms, TNF-α can also induce melanocyte dysfunction and cell death while promoting the progression of vitiligo [27]. In addition, TNF-α may facilitate the development of Tregs and play an anti-inflammatory role [28]. IL-1β represents a key product of inflammasome. IL-1β can downregulate MITF expression, which is associated with melanocyte differentiation, and inhibits melanocyte differentiation [29]. Previous studies have demonstrated that oxidative stress can induce an upregulation of IL-1β expression in the skin of vitiligo patients, with its levels positively associated with disease activity and severity [30]. Additionally, IL-6 acts as an inhibitor of melanocyte proliferation while also stimulating the expression of intercellular adhesion molecule-1 (ICAM-1) on melanocytes, thereby enhancing lymphocyte attachment to melanocytes, and facilitating melanocyte destruction [31, 32]. After CBI treatment, these cytokines exhibited a significant decrease in the responder group, while remaining elevated in the non-responder group. These data suggested that the associated proinflammatory response is not effectively controlled in the non-responsive cohort.
The current literature strongly supported the pivotal role of Th1 cytokines in the pathogenesis of vitiligo [11]. In this study, a significant increase in the levels of signature cytokines IFN-γ and IL-2 secreted by Th1 cells was observed among patients with active vitiligo. IFN-γ plays a pivotal role as a cytokine in the pathogenesis of vitiligo, exerting direct cytotoxic effects on melanocytes or driving CD8+ T cells to attack them, ultimately resulting in melanocyte destruction and subsequent skin depigmentation [11]. Previous investigations have indicated a significant elevation in the proportion of circulating skin-homing cells that produce IFN-γ among patients with vitiligo [5]. Furthermore, our findings demonstrated a positive correlation between BSA and IFN-γ levels, which is consistent with previous research indicating that elevated IFN-γ levels are associated with vitiligo disease severity [33]. In the pathogenesis of vitiligo, elevated IL-2 levels are thought to be closely related to lymphocyte activation and melanocyte destruction [34]. Several studies have consistently reported a significant increase in serum IL-2 levels among patients with vitiligo [20, 35]. After 3 months of CBI treatment, the circulating IFN-γ and IL-2 levels in the responder group were reduced to normal levels, while the non-responder group also experienced a decrease in serum levels to some extent. However, this reduction was not statistically significant compared to pre-treatment values, indicating inadequate control of the Th1 immune response in this group. In conclusion, these results suggested that the presence of circulating Th1 cytokines in vitiligo patients is closely related to disease progression, and part of the mechanism of CBI treatment may be to control disease progression by reducing the secretion of IFN-γ and IL-2.
IL-17, a pro-inflammatory cytokine secreted by Th17 cells, can stimulate the secretion of IL-6, TGF-β, and IL-1β among other cytokines. This cascade of events ultimately leads to the downregulation of melanocyte function-related genes MITF and aberrant cell morphology [36]. Furthermore, IL-17 facilitates the production of chemokine CCL20 and recruits cytotoxic CD8+ T lymphocytes into peripheral tissues [37]. Several clinical studies had demonstrated the limited efficacy of targeted IL-17 inhibition in vitiligo treatment, thereby suggesting a minor role for IL-17 in the pathogenesis of this condition [38, 39]. However, we also observed elevated levels of IL-17 in patients with active vitiligo, which exhibited a significant positive correlation with BSA. This finding is consistent with previous studies conducted on diverse ethnic populations and suggests the potential involvement of IL-17 in the pathogenesis of vitiligo [40, 41]. What is noteworthy is that the serum IL-17 level in the responder group was significantly reduced to a normal range following CBI treatment. However, the non-responder group still exhibited elevated levels of IL-17. This finding further supported the crucial role of IL-17 in vitiligo pathogenesis, and suggested that CBI treatment can partially mitigate disease progression by attenuating IL-17 levels.
We observed a significant decrease in IL-10 levels among vitiligo patients compared to the control group. IL-10, an anti-inflammatory cytokine secreted by Treg cells, exerts regulatory effects on immune responses and suppresses pro-inflammatory cytokines produced by activated T cells [42]. The efficacy of local tacrolimus and NB-UVB treatment in promoting repigmentation among vitiligo patients has been demonstrated by several studies, which also revealed a significant increase in the concentration of IL-10. These findings suggested that IL-10 may play a crucial role in facilitating skin pigmentation restoration [43]. The contradictory reports on IL-10 levels in patients with vitiligo across different studies may be attributed to variations in disease severity and individual differences [44]. Following CBI treatment, a significant increase in IL-10 levels was observed in the responder group, indicating that CBI treatment can enhance anti-inflammatory cytokine levels in vitiligo patients.
In recent years, numerous studies have reported heightened levels of chemokines such as IL-8 and CXCL10 in the serum and epidermis of vitiligo patients, corroborating our findings [45, 46]. IL-8, a crucial chemokine implicated in inflammatory skin disorders, is produced by monocytes, fibroblasts, dendritic cells, and keratinocytes [47]. Previous studies revealed that IL-8 can induce oxidative stress and subsequently trigger apoptosis of keratinocytes and melanocytes in vitiligo [48]. The chemokine CXCL10, induced by IFN-γ, plays a crucial role in promoting the migration of autoreactive CD8+ T cells to the skin and inducing melanocyte apoptosis [49]. Previous studies have demonstrated significantly elevated levels of CXCL10 in both vitiligo skin lesions and serum, with its expression being positively correlated with disease duration and infiltration level within the skin lesions. Furthermore, it has been identified as a potential predictor for therapeutic response [14]. In our study, we observed elevated levels of IL-8 and CXCL10 in patients with vitiligo, with a positive correlation between CXCL10 levels and BSA. Following CBI treatment, both the responder group and non-responder group exhibited significant reductions in serum levels of IL-8 and CXCL10, while the responder group achieved normalization of CXCL10. These findings suggested that CBI treatment has the potential to suppress the autoimmune response in vitiligo patients by reducing the levels of IL-8/CXCL10 chemokines, thereby facilitating repigmentation.
Our study suggested that vitiligo patients with elevated serum levels of TNF-α, IL-1β, IFN-γ, and IL-2 cytokines may exhibit increased resistance to CBI therapy, thereby impeding effective disease control. Further investigation is required to elucidate the role of heightened cytokine levels in vitiligo patients resistant to CBI treatment. These findings will contribute towards advancing our quest for more efficacious strategies in managing vitiligo progression.
The elevated levels of multiple cytokines in vitiligo suggested that its pathogenesis involves immune cell interactions mediated by multiple cytokines/chemokines. This may require a combination therapy or multi-target drugs to address resistance to glucocorticoid therapy in vitiligo [50].
Biologics represent a novel category of cytokine-based immunomodulators that hold promise as therapeutic alternatives for vitiligo patients resistant to conventional treatments. Pilot trials have demonstrated the efficacy of TNF-α inhibitors, including infliximab, etanercept, and adalimumab, in managing progressive vitiligo [51, 52]. Clinical trials investigating the efficacy of anti-IL-17A treatment in advanced vitiligo have yielded inconclusive results, necessitating further investigation into whether secukinumab can enhance combination therapy [38]. Treatment with CXCL10 neutralizing antibodies effectively reduced pigmentation and induced repigmentation in mice with vitiligo after a 4-week period [49]. Broad-spectrum Janus kinase (JAK) inhibitors exhibit a wide range of cytokine receptor signalling blockade and hold significant potential for the treatment of vitiligo [53]. Ruxolitinib, a selective JAK1/2 inhibitor, effectively suppresses downstream JAK-dependent cytokines such as IFN-γ, IL-1β, IL-6, TNF, CXCL9, and CXCL10 [54]. The topical formulation of ruxolitinib has demonstrated favourable efficacy and tolerability in the treatment of vitiligo, as confirmed by several large-scale clinical studies [55, 56]. Collectively, these studies provide evidence supporting potential therapeutic interventions targeting relevant cytokine axes. Drugs that target single or multiple cytokine axes have the potential to serve as effective treatments for patients with vitiligo and hormone resistance. It is noteworthy that vitiligo may manifest or progress during treatment with various biologics, such as TNF-α inhibitors, necessitating vigilant monitoring and assessment of the patient’s condition by the physician upon initiation of biologic therapy [39]. Furthermore, there is a pressing need to develop pharmacological agents that exhibit enhanced efficacy while minimizing adverse effects.
Previous studies have demonstrated minimal systemic absorption and negligible blood concentrations of tacrolimus following topical application of tacrolimus ointment [57, 58]. Similarly, the long-term use of halometasone has been associated with limited systemic effects [59]. However, the potential effects of topical halometasone and tacrolimus in our study cannot be completely ruled out.
The limitations of this study lie in the following points. First, the sample size was small and the follow-up time was short. Second, due to the difficulty of biopsy, we did not collect the lesions for cytokine level testing. Third, there was no in-depth analysis of the effects of topical drugs. It is necessary to verify the immunomodulatory effects of CBI in a larger sample size trial.
Conclusions
This study demonstrated that systematic application of CBI can effectively restore immune balance and repigmentation in active vitiligo patients by regulating the levels of pro-inflammatory cytokines, Th cells, and Treg cytokines. Elevated serum levels of TNF-α, IL-1β, IFN-γ, and IL-2 in patients with vitiligo before treatment may be a predictor of poor response to compound betamethasone injection.