Introduction
Bariatric surgery is the most effective treatment of obesity and related diseases [1]. Several procedures are currently offered to patients. Laparoscopic sleeve gastrectomy (LSG) is the most commonly used in North America and Europe [2–4]. Despite the significant clinical benefits from surgery some patients were disappointed with the weight loss due to unrealistic expectations. This leads to an abandonment of weight loss goals and may negatively influence the long-term outcome [5, 6]. Setting realistic expectations is an important aspect of the preoperative education. It is a key to successful treatment [5, 7]. However, there is currently no clinically useful tool to estimate the expected body mass index (BMI) in patients after LSG.
Aim
The purpose of this study is to develop a specific prediction calculator for estimation of the expected BMI at 1 year after LSG.
Material and methods
The data of 211 patients were retrospectively collected from medical records. All patients completed 1-year follow-up. Obese patients undergoing primary LSG from January 2011 to September 2015 were included. The outcome of the study was defined as the BMI at 1 year after the initial procedure. The independent demographic variables were: gender, race, age, preoperative body mass index, and smoking status. Examined comorbidities included diabetes mellitus type II, hypertension, obstructive sleep apnea, and dyslipidemia (Table I). Weight loss was expressed as change in BMI (ΔBMI), total weight loss (TWL), and percentage of excess weight loss (%EWL).
Ethical statement
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statistical analysis
Data were analyzed using SAS University Edition software (SAS Institute Inc., Cary, NC, USA). Least angle regression (LARS) was employed for variable selection. This new model selection algorithm, developed by Efron et al., relates to the classic model selection method known as forward selection but instead of including variables at each step, the estimated parameters are increased in a direction equiangular to each one’s correlations with the residual [8]. Initially, the BMI at 1 year after surgery (dependent variable) was transformed by logarithmic transformation. The data were then split into a training (65%) and testing set (35%). In addition, the external data set was employed for external validation. The model was developed using a hybrid method and the average square error (ASE) over the validation data as a selection criteria. Goodness of fit was assessed by ASE. To test the accuracy of the predictive model, the Wilcoxon signed-rank test was performed between estimated BMI and BMI observed 1 year after surgery. A linear logistic equation was used to construct the online predictive calculator (www.predictbmi.com). It is also available as the app for iOS.
Results
Table I presents the descriptive characteristics of 211 patients. The median age of patients was 45 (Q1: 38, Q3: 54) years and median BMI was 45.3 (41.2, 52.2) kg/m2 at the time of surgery. The median postoperative BMI was 33.6 (29.4, 38.8) kg/m2. The median %EWL was 58.6 (43.6, 73.3) %. The median ΔBMI was 13.5 (10.2, 17.5) kg/m2 and the median TWL was 32.7 (25.4, 43.5) kg (Table II). Sixty-three percent of patients achieved EWL > 50% at 1 year after surgery (Table III).
Table I
Independent variables included to the analysis
Table II
Weight loss outcome in studied sample
Table III
Excess weight loss categories in studied sample
| Excess weight loss category | N (%) |
|---|---|
| EWL < 30% | 19 (9) |
| 30% < EWL < 50% | 59 (28) |
| EWL > 50% | 133 (63) |
Model development
Nine variables were analyzed. The model included three variables: age, preoperative BMI and gender (Table IV). All predictors were significant: preoperative BMI (β = 0.023, p < 0.001), age (β = 0.005, p < 0.001), and female gender (β = 0.116, p = 0.001).
Table IV
Comparison of observed and estimated BMI after surgery in studied sample (n = 211)*
| 1-year postoperative BMI | Mean ± SD | Median (IQR) |
|---|---|---|
| Observed | 34.8 ±7.6 | 33.7 (9.4) |
| Estimates | 34.9 ±6.9 | 33.0 (9.0) |
The linear regression equation was as follows: logarithm BMI = 2.111 + (0.005 × age) + (0.023 × preoperative BMI) + (0.116 × female gender).
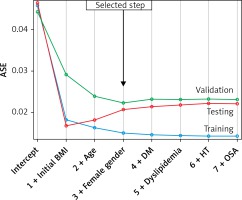
The model was significant (p < 0.001) and explains 67% of weight loss (R 2 = 0.672; adjusted R 2 = 0.664). The root of the mean standard error of the estimate was 0.124. Figure 1 shows the progression of the ASE separately for the training, validation, and test data. The desirable behavior is present where the ASE for the training, validation, and test data all decrease monotonically with the selection terminating at the step beyond which the test and validation errors would begin to grow. The difference in ASE between training and testing data sets was –0.005 and that between training and validation data sets was –0.007. The median estimated BMI was 33.0 (30.0, 39.0) kg/m2. The difference between the observed BMI and the estimated BMI was not statistically significant (median = 0.737 (–2.676, 3.254); p = 0.223).
External validation
The validation sample included 184 patients. All patients underwent LSG as a primary bariatric procedure in a European high volume bariatric center between January 2013 and December 2015. 55% were female. Median age was 38.5 (31.5, 48.0) years. The median BMI was 46.7 (41.6, 52.1) kg/m2. The median BMI at 12 months after surgery was 31.3 (26.9, 35.2) kg/m2. The median %EWL was 71.6 (58.6, 88.6) %. The median ΔBMI was 14.9 (12.0, 18.9) kg/m2 and the median TWL was 43.5 (35.0, 56.6) km. 87.5% of patients achieved EWL > 50% at 1 year after surgery. The median expected BMI estimated by the model was 32.0 (28.0, 36.0) kg/m2. The difference between the 1-year post-surgery estimated BMI and the BMI observed 1 year after surgery was not statistically significant (median: –0.223 (–3.506, 2.667); p = 0.160). The final model accurately predicted the 1-year post-surgery BMI.
Online calculator
The linear regression equation was the basis for development of the online calculator called PREDICT BMI. It provides information about expected BMI and weight loss at 1 year after surgery. Weight loss is calculated indirectly from expected BMI. A free version of the calculator is available at http://www.predictbmi.com.
Some examples of the expected BMI using the PREDICT BMI are as follows:
A 32-year old female patient with BMI 46.9 kg/m2. Expected BMI at 1 year after surgery would be 28.9 kg/m2.
A 30-year old male patient with BMI 37.0 kg/m2. Expected BMI at 1 year after surgery in 22.5 kg/m2.
A 54-year old female patient with BMI 46.3 kg/m2. Expected BMI at 1 year after surgery would be 31.9 kg/m2.
Discussion
Bariatric surgery is a very effective treatment of obesity and related comorbidities [9].
Due to the rising prevalence of obesity, bariatric surgery has become very popular in many countries. Several procedures are currently offered to bariatric patients. The LSG is the most popular [2]. The method is relatively safe and feasible as a primary and revisional procedure [10, 11]. In spite of high clinical efficacy, many patients are disappointed with the effects. The expectations regarding weight loss after surgery are greater than the results expected by the surgeons [5]. Communication between the patient and the bariatric surgeon is very important as it influences the whole treatment process. Setting realistic expectations may be the key to success [7].
We proposed a prediction model of the BMI at 1 year after surgery in patients who are scheduled for LSG. The predictive model was developed using an LARS algorithm. This new statistical approach is a better option for variable selection than the widely used stepwise regression or similar selection methods which are not recommended in the literature [12]. The presented model included three variables. Age, preoperative BMI and female gender were associated with the BMI at 12 months after the procedure. All predictors were statistically significant in analysis of variance. The model was validated on an external dataset and showed good performance. The simplicity of the proposed model makes it easy to use. On the basis of the model we developed a calculator, which can be used for estimation of weight loss. Using this calculator a bariatric surgeon can set a realistic expectation regarding weight loss.
Previous studies focused on predictive factors for weight loss after bariatric surgery and proved the significance for all factors included in the model [13–16]. The negative correlation between preoperative BMI and weight loss has been well described [17]. There is evidence for a negative association between age and weight loss after surgery [14, 15, 18–20]. Female gender was found in many studies to be a negative predictor for weight loss after surgery [15, 19, 21, 22].
The number of studies presenting prognostic models for expected weight loss after surgery is limited. Goulart et al. presented the same idea for a prognostic model for bariatric patients. Their model was developed based on data derived from 152 patients after LSG and included age and preoperative BMI. That model explained 64% of weight loss [23]. However, the model was not validated on an external dataset.
The presented study has several limitations. First, the variability in bariatric patients is high.
In order to make a simple model and avoid overfitting, we limited the number of factors in our analysis. We did not include in variable selection important predictors such as onset of obesity, socioeconomic status, functional status, eating habits and psychological disorders.
Second, the weight loss is affected by surgical factors such as the bougie size, distance from pylorus and the completeness of resection of the fundus. Those factors were not included in the model. Inclusion of the above factors would make it more complex. Some of them would also be difficult for objective assessment.
Third, the calculator addresses the issue of expected weight loss, but many patients have unrealistic expectations about their body image after surgery [23]. Thus, the estimation should be followed by a physician’s comment about that.
Finally, there is a certain risk of misinterpretation of estimations. Thus, we recommend using the calculator for setting a weight loss goal after surgery and informing the patient that the final result depends on his compliance with postoperative recommendations regarding diet and physical activity.
Despite those limitations, the PREDICT BMI calculator can be very useful in clinical practice. This user-friendly tool allows one to answer an important question asked by the patient: “How much weight will I lose?”. The estimation of postoperative BMI and expected weight loss provides the answer and can be useful for motivational purposes.
Conclusions
We have presented a predictive model for estimating BMI at 1 year after LSG. The model includes the following factors: age, gender, preoperative BMI. The PREDICT BMI calculator developed based on this model allows for the estimation of patients’ BMI at 1 year after surgery and the setting of realistic expectations. It is essential to emphasize that the estimation is only indicative and the final result is dependent on the patient’s adherence do dietary and physical activity recommendations after surgery. The ability to set realistic expectations is the key for satisfaction after surgery. Once a realistic goal is set, the patient can be encouraged to attain it.