PURPOSE
The World Health Organization (WHO) approximates that after the acute phase of SARS-CoV-2 infection, 10-20% of individuals infected with the virus report experiencing persistent or newly emerging symptoms during the extended course of illness. This condition is commonly referred to as post-COVID syndrome [1]. The most frequent neurological complaints observed in individuals post-SARS-CoV-2 infection include fatigue, difficulties with concentration and memory, headaches, vertigo, myalgia, neuropathy, and persistent alterations in the sense of smell and taste. Moreover, there have been reports of disruptions in autonomic functions, and neurological conditions have been documented as sequels following COVID-19. Notably, within the initial year after the acute SARS-CoV-2 infection, there is a reported 50% increased risk of vascular-related disorders, such as stroke and transient ischemic attacks.
Epileptic seizures, myelitis, as well as peripheral neurological disorders such as Guillain-Barré syndrome, cranial nerve deficits, myositis, and plexopathies have also been documented. In rare instances, cases of autoimmune encephalomyelitis have been reported three months post-COVID-19 infection. The precise underlying mechanism responsible for post-COVID syndrome remains unclear; however, it is widely believed that immune dysregulation plays a central role in many of these cases [2].
Sarcoidosis is a multi-organ disease of unknown origin characterized by the presence of granulomatous inflammation in affected organs. The organs most commonly affected include the lungs, skin, eyes, and liver. Additionally, approximately 10% of cases manifest as neurosarcoidosis, indicating the involvement of the nervous system. While the precise cause of sarcoidosis is not fully understood, it is postulated that infectious agents may contribute to its development.
Neurosarcoidosis represents one of the rarest neurological complications that can occur in individuals who have contracted COVID-19. Clinical presentations in neurological cases tend to be diverse and typically lack specific characteristics. Cranial neuropathy can result in minor abnormalities in protein and cell counts. Approximately half of the cases exhibit nerve enhancement in imaging studies, whereas others may show no detectable abnormalities [3]. In this manuscript, we explore an unusual case of post-COVID neurosarcoidosis. The investigation of this case may potentially bolster the association between COVID-19 and neuroinflammation.
CASE DESCRIPTION
A 51-year-old woman presented at our tertiary medical center with a persistent throbbing non-positional holocranial headache, along with recurring nausea, non- hemorrhagic vomiting, and vertigo over the previous eight months. These symptoms began one month after her hospitalization for COVID-19. One month prior to referring to us, she developed right-eye esotropia, restricted eye movement in the same eye, mild left hemiparesis, dysphagia, and ataxia. Before this medical evaluation, she had not received a comprehensive neurological assessment and had been taking acetaminophen at a daily dose of 1 mg, which had not improved her condition.
During the clinical examination, the patient appeared unwell but not severely so. She was awake, alert, cooperative, and had normal speech. Her vital signs were within the normal range, and she did not have a fever. Visual assessment showed normal visual acuity with no visual field abnormalities in both eyes. Her pupils reacted normally, and there was no papilledema. The gag reflex was mildly increased, and both patellar reflexes were hyperactive. Sensation was normal, muscle tone was as expected, and there was 4/5 strength in the left lower extremity and 5/5 strength in the right lower extremity. Deep tendon reflexes were increased (+2).
The patient worked as a housekeeper and declared that she had not traveled recently or had contact with sick individuals. She had a medical history of type 2 diabetes mellitus and hyperlipidemia and reported no history of primary or similar headache. Her current medications included metformin, atorvastatin, and aspirin. There was no significant family history of note.
Investigations
A brain magnetic resonance imaging (MRI) with gadolinium, performed during the patient’s hospitalization, revealed multiple small hyperintense lesions on the T2 and fluid-attenuated inversion recovery (FLAIR) sequences in the white matter of both cerebral hemispheres, as well as in the medulla, pons, lateral hypothalamus, and middle cerebellar peduncles. Pathological contrast enhancement was observed, but there were no signs of diffusion restriction or leptomeningeal enhancement.
A cervical MRI with gadolinium demonstrated a long- segment T2 hyperintensity with mild pathological contrast enhancement (Figures I and III).
Figure I
A sagittal T2-weighted sequence MRI of the brain stem and cervical and upper thoracic spine showing (A) hypothalamus involvement, (B) inferior pons involvement, and (C) inferior medulla and C1 to C5 vertebrae
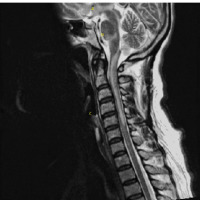
Figure II
A sagittal T2-weighted sequence MRI of thoracic vertebrae showing lower thoracic involvement
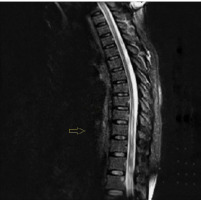
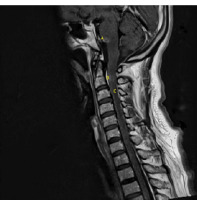
Figure III
A sagittal T1-weighted contrast-enhanced MRI of the brain stem and cervical and upper thoracic spine showing (A) post-contrast enhancement of inferior pons, (B) faint enhancement of upper cervical, and (C) meningeal enhancement
In a thoracic MRI with gadolinium, a few small areas of T2 hyperintensity with contrast enhancement were noted. Small nodules were observed in the breast and chest, although a biopsy was not performed due to their small size, and a follow-up plan was established (Figure II).
Given the progressive nature of these findings, there was a strong suspicion of meningitis. Infectious disease consultants recommended a lumbar puncture after the patient’s admission to the hospital. Cerebrospinal fluid (CSF) analysis revealed clear fluid with a protein level of 201 mg/dl, glucose level of 118 mg/dl, a white blood cell count of 100/µl (consisting of 40% lymphocytes and 60% neutrophils), and no evidence of malignant cells or oligoclonal bands.
Based on the recommendations of the infectious disease consultants, antibiotic therapy was initiated. However, it was discontinued after the CSF culture analysis yielded negative results, and the diagnosis was revised to aseptic meningitis. A further panel PCR test for the nine most common neuroviral infections, and autoimmune panel tests, including for rheumatoid factor (RF), antineutrophilic cytoplasmic antibody (ANCA), perinuclear ANCA (P-ANCA), cytoplasmic ANCA (C-ANCA), myelin oligodendrocyte glycoprotein (MOG), and neuromyelitis optica (NMO) antibodies all returned negative results. The CSF culture for a Mycobacterium tuberculosis investigation also came back negative.
Due to elevated CSF pressure (25 mmHg), acetazolamide was prescribed at a daily dose of 1 g, which partially alleviated the patient’s headache. The CSF level of angiotensin-converting enzyme (ACE) antibody was elevated, suggesting the possibility of neurosarcoidosis. After ruling out other potential differential diagnoses, neurosarcoidosis was considered as the most probable diagnosis.
Differential diagnosis
Clinical evaluations decreased the possibility of carcinomatous meningitis, other autoimmune diseases, malignancies, vasculitis, common viral and microbial infections, and occupational toxifications and the differential diagnoses of the presented case. In most cases, the appearance of lesions is nonspecific so that sarcoidosis is included in a broad differential diagnosis. It is necessary to note that sarcoidosis lesions may resemble brain tumors, especially meningioma, so a histopathology analysis plays an essential role in the best diagnosis. Other differential diagnoses for the imaging findings include nerve sheath tumors, lymphoma, carcinomatous metastasis, chloroma, hemangiopericytoma, and other granulomatous depositions [4].
Treatment and outcome
The patient underwent treatment with intravenous methylprednisolone injections at a daily dosage of 1 g for a period of five days, which was subsequently followed by oral prednisolone at a daily dose of 50 mg. This regimen led to a limited improvement in the patient’s condition. Despite this, concerns related to left hemiparesis persisted, prompting the implementation of a follow-up plan to monitor the lesions (Figure IV).
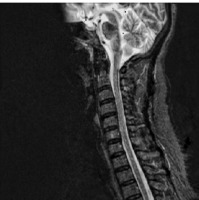
Figure IV
A sagittal T2-weighted MRI of the brain stem and cervical spine showing the elimination of all the lesions after pulse therapy with corticosteroids
After a duration of two months, notable improvement has been observed in the patient’s symptoms. Nevertheless, occasional episodes of headache continue to be a persistent issue.
Comment
Considering the disease’s progression, which initially manifested as aseptic meningitis followed by the emergence of CNS lesions, the most likely diagnosis appears to be neurosarcoidosis. To definitively confirm this diagnosis, a biopsy of the affected tissue is required to demonstrate non-caseating granulomas, a hallmark of sarcoidosis. However, in clinical practice performing a brain biopsy is often impractical and reserved as a last resort. Consequently, a diagnosis of neurosarcoidosis is typically established based on clinical criteria, including consistent symptomatology, laboratory findings from both blood and CSF analyses, radiological assessments, and the exclusion of other potential diagnoses [5]. In this particular case, due to the absence of specific findings on the chest computed tomography scan, the unavailability of a positron emission tomography scan, and the impracticality of performing biopsies on the affected regions of the CNS, it has not been possible to establish a definitive diagnosis of sarcoidosis.
Nevertheless, sarcoidosis remains a plausible diagnosis, primarily due to the elevated levels of ACE antibody. ACE serves as a suitable indicator of systemic granulomatous processes, as it is known to be associated with granulomas throughout the body [6]. It is important to acknowledge, however, that other autoimmune conditions, such as neuromyelitis optica spectrum disorder and autoimmune vasculitis, which can be precipitated by a COVID-19 virus infection, should be taken into consideration as potential differential diagnoses in this case [4, 7]. In this study, we have tried to rule out these conditions as well as the most common autoimmune diagnoses by using a comprehensive panel of autoantibodies.
Sarcoidosis is an inflammatory condition characterized by an exaggerated cell-mediated, granulomatous response to antigens that have not yet been identified. While there is no consensus on whether sarcoidosis should be classified as an autoimmune disease, there is a growing body of evidence suggesting that autoimmunity may play a role in certain forms of the condition. Our current understanding of sarcoidosis pathogenesis primarily stems from research conducted on pulmonary sarcoidosis. However, it remains an active area of investigation to determine the extent to which these findings apply to neurosarcoidosis and whether the immunological processes in NS differ from those in sarcoidosis affecting other organ systems.
It has been theorized that infectious agents may influence the development of sarcoidosis. These agents could either directly induce a granulomatous inflammatory response or indirectly affect the epigenetic and immunological changes associated with sarcoidosis. Among various infectious agents, mycobacteria and Propionibacterium acnes have been shown to have the most significant links to sarcoidosis in terms of potential associations [8]. Indeed, there have been a few reports suggesting a potential link between COVID-19 and neurosarcoidosis [9]. The pathophysiology of headache in the context of long COVID-19 involves multifactorial mechanisms. Studies suggest that the prevalence of post-COVID headache remains consistent across patients with severe and non- severe forms of the disease, with higher occurrences among those initially managed in outpatient settings during the acute phase. SARS-CoV-2 can trigger headache through trigeminovascular system activation in patients with a genetic predisposition to migraine or pre-existing headache, hypoxia, hypercapnia, structural and functional brain changes, and most importantly, immune system activation. COVID-19 represents a complex interplay of biohumoral responses with evident alterations in cytokines and interleukins that can lead to headache. Studies have reported varied immune responses in individuals experiencing persistent headaches post-COVID infection. Notably, lower levels of interleukin-6 were observed in some studies, while others indicated higher mean levels, particularly in patients with bilateral headaches. Additional biomarkers such as HMGB1, NLRP3, angiotensin II, and ACE2 exhibited elevated serum levels in individuals with COVID-19-related headaches. Conversely, an alteration in IL-10 blood levels has been noted, reflecting the nuanced immune profile associated with Long COVID headache. The specific impact of immune system activation over time in patients with predisposing headache biology remains a topic of ongoing investigation. A notable finding from a multivariate analysis of hospitalized patients followed for one year revealed that immune-compromised individuals experienced a prolonged duration of headaches, emphasizing the role of the immune response in the persistence of post-COVID headache symptoms [10].
The objective of disease-modifying treatment for neurosarcoidosis is to mitigate or prevent damage to organ systems resulting from the adverse effects of granulomatous inflammation. In the comprehensive care of patients with neurosarcoidosis, it is essential to include therapies aimed at managing symptoms and facilitating rehabilitation. As of now, there are no randomized trials available to provide precise treatment guidelines for neurosarcoidosis. Consequently, treatment decisions are primarily informed by expert opinion and insights drawn from case series and individual reports.
In general, glucocorticoids constitute the standard first-line treatment for neurosarcoidosis. In cases of more severe disease manifestation, initial treatment may involve intravenous (IV) or oral (PO) bioequivalent pulse dosing, such as a course of 1 gram IV methylprednisolone administered over 3-5 days. This is typically followed by a gradual tapering of oral glucocorticoid therapy over several months (e.g., prednisone at a dose of 1 mg/kg/day, with gradual reduction), with the speed and duration of the taper being adjusted based on factors such as disease severity, risk, patient tolerability, and clinical and imaging response [11]. The substantial improvement observed in our patient following the administration of oral steroids, coupled with the subsequent resolution of CNS lesions in follow-up examinations, provides additional support for the likelihood of a sarcoidosis diagnosis.
Our case also implies a potential correlation between COVID-19 and the onset of sarcoidosis, characterized by notable clinical presentations. Currently, the existence of such an association remains uncertain and necessitates additional extensive observations for conclusive determination.







