Arterial calcifications are a common challenge during coronary interventions. They can cause problems with proper stent expansion and hence with good long-term results of the intervention [1], and with obtaining a safe access if they are located peripherally. This is especially important in procedures involving large bore access [2], but in some cases they can also disrupt routine coronary angiography.
A 78-year-old male with a history of atrial fibrillation was admitted due to recent exacerbation of his symptoms – atypical, stabbing chest pain after light exercise and while sometimes resting. Due to reduced left ventricle ejection fraction (45%) and segmental hypokinesis in transthoracic echocardiography, the patient was qualified for a coronary angiography. The procedure was initiated with a radial puncture to acquire arterial access. After puncturing the artery and difficult insertion of a wire, it was not possible to introduce a standard 6F introducer sheath; therefore, a hydrophilic-coated Glidesheath Slender (TERUMO) introducer sheath was applied instead. A standard 0.035” wire or hydrophilic J-Tip Glidewire (TERUMO) could not be advanced through the radial artery. The artery was passed with a coronary 0.014” Sion Blue (ASAHI) guidewire, which allowed slow advancement of a 5F diagnostic catheter through the radial artery with a great resistance. Forearm angiography revealed patent radial and ulnar arteries, with massive intramural calcifications, uniform in the whole course of the arteries (Figure 1 A). The coronary wire was changed back to 0.035”, which reached the subclavian artery through the catheter; however, it was not possible to advance the catheter any further due to great resistance. Because the catheter would have no manoeuvrability upon hypothetically reaching of the coronary sinus, it was changed to a 6.5F Sheathless Eucath (ASAHI) hydrophilic-coated catheter. Nevertheless, the Sheathless catheter was not able to cross the radial artery with the support of a 0.035” wire, and the attempt to advance it ended in an inversion of the introducer sheath, with damage to the haemostatic valve (Figure 1 C). Therefore, a crossover to the femoral access was performed. The femoral artery was punctured, and the 0.035” guidewire was introduced to the abdominal aorta. Due to the stiffness of the femoral artery wall, an attempt to insert a 6F sheath resulted in damage to the sheath dilator (Figure 1 D). For safety reasons, new sheaths with dilators were used for subsequent attempts, to avoid damage of the vessel or distal embolisation. Upon the fifth attempt, a reinforced 6F sheath was successfully introduced. Coronary angiography via the femoral access revealed no significant narrowings or calcifications. The femoral puncture site was closed with a standard pressure dressing a few hours after the procedure, because calcifications impair the result of vascular closure device application [2]. After sheath removal, a large haematoma appeared at the site of the femoral puncture (Figure 1 E).
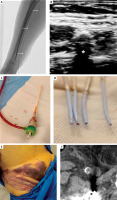
Figure 1
A – X-ray image of forearm demonstrating severe calcifications in radial, ulnar, and brachial arteries (marked with arrows); B – ultrasonogram of heavily calcified radial artery, anterior and posterior wall marked with arrows, acoustic shadow marked with an asterisk; C – Slender sheath damaged due to stiffness of the radial artery; D – femoral sheaths damaged during subsequent attempts of insertion into severely calcified artery E – large superficial haematoma in the puncture site – a result of ineffective haemostasis in a stiff vessel; F – computed tomography revealing severe intramural calcification in femoral artery, marked with an arrow. Asterisk indicates an artifact caused by an endoprosthesis
Calcium, phosphates, and parathormon levels, measured in search of the reason for such severe calcifications, were within normal limits. Angio CT revealed circular intramural calcifications restricted to the brachial artery and its branches, as well as the femoral artery (Figure 1 F) and its distal branches. The affected segments were patent, without significant narrowings. The patient was discharged 2 days after the procedure and will undergo further diagnostics to find the cause of the calcifications.
There are several methods of management in cases of difficulty in passing the radial artery, involving hydrophilic-coated devices and coronary guidewires. However, in rare cases they may not be sufficient due to extreme stiffness of the calcified artery. Ultrasound should be performed before radial puncture because it allows for quick and feasible assessment of the artery [3], which should not be punctured in the case of severe calcifications. Calcifications in the puncture site greatly impair haemostasis.








