Summary
Long-term durability of carotid artery stenting in terms of incidence of recurrent restenosis is a poorly examined issue. Our results suggest that the risk of restenosis is strongly associated with bilateral and high-grade carotid artery stenosis as well as with initial Carotid Wallstent implantation. These findings may influence the procedure strategy in patients with carotid artery stenosis.
Introduction
Carotid artery stenting (CAS) has emerged as a strong alternative to carotid endarterectomy (CEA) in patients with carotid artery stenosis. Fast development of endovascular techniques and increasing operators’ experience have resulted in significant reduction of the periprocedural complication rate during CAS [1]. On the other hand, the growing number of CAS procedures performed around the world entails enlarging population of patients with in-stent restenosis (ISR) defined as reoccurrence of stenosis within stent. The prevalence of ISR after CAS ranges from 4.6% to 6.3%, with half of cases occurring within the first 6 months [2]. There are many ISR risk factors established; they include history of prior ipsilateral neck surgery or irradiation (‘hostile-neck’ lesions), diabetes, female sex, and dyslipidemia [3–5]. Interestingly, the brand of stent has not yet been investigated as a potential risk factor. Although ISR accompanies endovascular carotid stenosis treatment from the very beginning, there are no well-defined guidelines on how to deal with this issue. Both conservative and interventional approaches have their supporters. Although ISR is usually asymptomatic, Donas et al. showed that ISR may be associated with thrombus formation and increased risk of thrombovascular events [6].
Moreover, 2-year follow-up in CREST revealed that patients who had restenosis within 2 years were at greater risk for ipsilateral stroke after the periprocedural period than were those who did not have restenosis [7].
The endovascular approach to ISR includes balloon angioplasty alone, cutting-balloon angioplasty, drug-eluting balloon angioplasty, bare-metal and drug-eluting stent angioplasty [8–11]. Long-term durability of carotid artery stenting in terms of incidence of recurrent restenosis is an even less examined issue. The therapeutic approach to first restenosis may influence both the immediate result and further restenosis reoccurrence.
Aim
The aim of the study was to evaluate the safety of different methods of endovascular treatment of carotid in-stent first/recurrent restenosis and to establish its rate and risk factors.
Material and methods
Study population
Between January 2001 and June 2016, 2637 neuroprotected carotid artery stenting procedures were performed in 2443 patients (men: 67.0%; mean age: 67.9 ±8.8 years, range: 33–90, symptomatic: 45.5%), according to the ‘tailored-CAS’ algorithm [12]. Patient characteristics are shown in Table I.
Table I
Characteristics of 2443 patients undergoing 2637 tailored CAS procedures
[i] Continuous data are presented as means ± standard deviation; categorical data are given as counts (percentages). CAS – carotid artery stenting, ICA – internal carotid artery, CCA – common carotid artery, TIA – transient ischemic attack, CEA – carotid endarterectomy, CAD – coronary artery disease. *Within 6 months prior to CAS. ‡Coronary artery lesion(s) ≥ 50% by quantitative angiography.
Ultrasound evaluation
Neurological and Doppler ultrasound evaluation (DUS) were performed at discharge, 3–6, 12 months after the procedure, and then annually. All DUS examinations were performed in a certified laboratory using a linear 7–10 MHz probe to evaluate the degree of ISR and they were evaluated according to international standards. Restenosis was defined as ≥ 50% diameter reducing stenosis (or occlusion). The peak systolic velocity (PSV) criteria used to evaluate the degree of ISR were 170–299 cm/s for 50–69% stenosis and > 300 cm/s for ≥ 70% ISR. PSV was measured within, distally and proximally to the stent. The highest PSV values were included in the analysis [13, 14]. The minimal time of observation was 12 months.
Endovascular approach
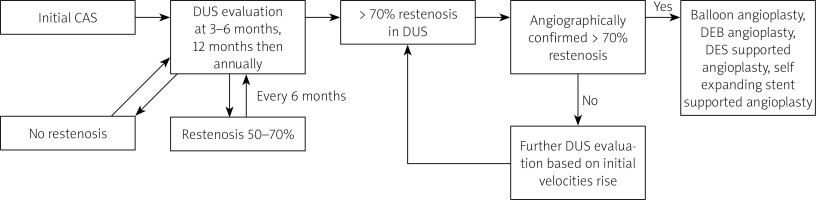
Balloon (non-covered until 2012, drug covered afterwards) angioplasty alone was performed in patients with angiographically confirmed ≥ 70% ISR. The balloon diameter corresponded to the distal internal carotid artery (ICA) reference. For diffused restenosis, extending outside the stent, implantation of another stent was considered. Subsequent, recurrent ≥ 70% ISR was treated with drug-eluting balloon (DEB), coronary drug-eluting stent (DES) implantation or self-expanding paclitaxel covered coronary stent supported angioplasty. The study flow chart is shown in Figure 1.
Definitions
– Closed-cell design stent – characterized by interconnected stent struts with small free cell areas (< 5 mm2),
– Distal neuroprotection – filter system used for capturing embolic material during carotid artery stenting with maintained ICA antegrade blood flow,
– Intolerance – cerebral ischemia symptoms occurring as a result of ICA flow blockage during neuroprotection/angioplasty balloon inflation,
– Neuroprotected carotid artery stenting – stent-supported carotid artery angioplasty using distal or proximal neuroprotection system,
– Proximal neuroprotection – system providing ICA blood flow cessation/reversal by common carotid and external carotid balloon occlusion during CAS,
– Restenosis – ≥ 50% diameter reducing in-stent stenosis (or occlusion) evaluated in ultrasound examination,
– Symptomatic patient – with history of ischemic stroke/transient ischemic attack (TIA), within preceding 6 months,
– ‘Tailored-CAS’ – patient and lesion adjusted selection of neuroprotection system and stent type; preferential use of proximal neuroprotection and close-cell stent for high-risk lesions (> 95% stenosis, thrombus-containing lesions, ’soft’ lesions, i.e. with computed tomography density of < 60 HU) and in symptomatic patients.
Ethics
This study was approved by the Committee on Research Ethics at our hospital, in compliance with the ethical guidelines of the Declaration of Helsinki. All participants gave written informed consent prior to study entry.
Statistical analysis
Continuous data were presented as means ± standard deviation; categorical data were given as counts (percentages). Normality of distribution was assessed by Kolmogorov-Smirnov test. The Χ2 test was used for comparison of categorical data. Potential risk factors included sex, age, smoking status, hypertension, hypercholesterolemia, prior stroke/TIA, prior ipsilateral CEA, contralateral stenosis/occlusion, stenosis grade, peripheral artery disease, stent type (open- vs. closed-cell design), stent brand and stent underexpansion defined as post-CAS stenosis of > 30%. In the case of repeated angioplasty due to restenosis, follow-up procedures were then conducted the same way as after initial angioplasty.
Univariate and multivariate Cox proportional hazard regression models were used to determine risk factors for the development of the first and recurrent restenosis. For each potential risk factor, the hazard ratio and associated 95% confidence interval from univariate analysis was examined. Multivariate analysis was then conducted with a logistic regression model. A p-value of < 0.05 was considered as statistically significant. All analyses were evaluated in Statistica 9.0 (StatSoft Inc).
Results
Demographic and clinical data
Out of 2637 stented arteries, in 95 cases (95/2637; 3.6%) > 50% ISR restenosis was detected in DUS. Of those, 53 (53/2637; 2.0%) patients had ≥ 70% restenosis confirmed in angiography, including one case of asymptomatic total occlusion. No stent fracture was detected. Mean PSV was 221 ±41 cm/s and 427 ±87 cm/s for 50–69% and ≥ 70% restenosis respectively (p < 0.001). All 52 patients (age: 49–77 years, 37 men, 19 patients symptomatic before initial CAS, time from initial CAS to first restenosis mean 22 ±27 months, 56% cases within first year, 22% within second year) were treated successfully by the endovascular approach.
Angiographic data
ISR treatment included bare (n = 19), DEB (n = 27) or DES supported (n = 6, all for diffused restenosis) angioplasty. In 7 cases cutting-balloon use was necessary, as the standard balloon was slipping out of the stent. In the DEB group there were 11 (11/27; 41%) cases of intolerance (cerebral ischemia) requiring shortening of inflation to < 40 s. The success rate was 100%; however, in 2 patients we were not able to cross a stent with distal embolic protection device (EPD) while proximal EPD was not used due to the risk of system intolerance. Angioplasty in those two cases was then non-protected. One (1.9%) procedure performed with distal EPD was complicated with ipsilateral ischemic stroke that occurred just after procedure completion. Angiographic diameter stenosis (DS) was reduced from 83 ±8.3% to 13 ±7.6% (p < 0.001). Characteristics of stents used for initial angioplasty and the restenosis rate in each stent group are shown in Table II. The treatment strategies for recurrent restenosis are shown in Table III.
Table II
Numbers and percentages of stents used for initial angioplasty and restenosis rate in each stent group
Table III
Technical details of in-stent restenosis (ISR) endovascular treatment
| Treated with | First ISR | Second ISR | Third ISR |
|---|---|---|---|
| Uncovered balloon angioplasty | 19/52* (36.5%) | ||
| DEB angioplasty | 27/52 (50.9%) | 4/13 (30.8%) | 2/3 (66.7%) |
| Self-expanding carotid stent | 6/52 (11.5%) | ||
| Balloon mounted coronary DES | 8/13 (61.5%) | ||
| Self-expanding coronary DES | 1/13 (7.7%) | 1/3 (23.3%) |
Follow-up
There were 13 cases (13/52 – 25%; age 52–77 years, 8 men) of ≥ 70% recurrent ISR; all were neurologically asymptomatic. The mean time to second in-stent restenosis was 26 months in the bare balloon group and 23 months in the DEB group (p = NS). The prevalence of recurrent restenosis in the non-covered balloon group was 32% (6/19) vs. 23% (6/26) in the DEB group (p = NS).
Excluding 2 cases of balloon-mounted DES collapse (implanted in the way that they extended from the initial stent due to edge restenosis), there were no further restenoses in the DES group in 20-month follow-up. Further cases of edge restenosis were treated with self-expanding coronary stent. Three patients (3/52 – 5.8%) required a third intervention, and 1 patient with Takayasu arteritis required five interventions.
Bilateral and high-grade stenosis were independent risk factors of restenosis (OR = 2.95, 95% CI: 1.87–4.64, p < 0.001 and OR = 1.9, 95% CI: 1.0–3.63, p = 0.049 respectively). Initial Carotid Wallstent implantation was a risk factor of first and recurrent in-stent restenosis (vs. combined group including all other stents, OR = 2.71, p < 0.001 and OR = 3.11, p = 0.032 respectively). Combining the three risk factors together (i.e. bilateral stenosis, high-grade stenosis and Carotid Wallstent implantation) significantly increases the odds ratios of a restenosis to 6.19 (95% CI: 2.90–13.20, p < 000.1). Stent underexpansion (n = 13) did not increase the risk of restenosis.
Discussion
Despite the limitations of DUS, associated with different biomechanical characteristics of native and stented artery, it is the best tool for in-stent restenosis detection. There are at least several different cut-off criteria proposed, based on PSV, end-diastolic velocity and PSV ratio. We decided to adopt the criteria of Lal et al. [13], as they showed a good correlation with angiological findings in detecting severe restenosis in our institution. Only 2 of 53 arteries with suspected ≥ 70% DUS-detected restenosis revealed borderline (40–70%) lumen narrowing in angiography.
Before the era of DEB, the carotid ISR was treated mainly with non-covered balloons with good immediate and long-term results. Our small sample of recurrent restenosis suggests that use of DEB does not significantly prolong the time for the next intervention as compared with non-covered balloons.
There are data showing that restenosis may be symptomatic and that it may be associated with significant risk of distal embolization during angioplasty for in-stent restenosis [6, 9, 10, 15]. This was a reason for the interventional approach to restenosis in our institution. Our results show that endovascular treatment of in-stent restenosis using neuroprotection is effective and safe, which also relates to patients treated for recurrent stenosis. The only case of periprocedural stroke was probably associated with mobilization of plaque fragments during catheter maneuvering and balloon inflation.
To date, there are no well-defined guidelines that establish the most effective approach for ISR treatment. While different endovascular strategies have been reported, mainly with uncovered and covered balloons, none of them may be applied in each patient.
The main disadvantage of DEB use is the necessity of prolonged inflation for optimal drug delivery. Transient, balloon-related flow stoppage in the treated artery may provoke cerebral ischemia, especially in patients with coexisting contralateral occlusion. In our DEB group there was a high (40.7%) percentage of neurological ischemic symptoms occurrence requiring shortening of the time of inflation to < 40 s. However, similar risk of, and time to, subsequent restenosis reoccurrence between uncovered balloon and DEB groups may only be an accidental finding as there are no robust data comparing results of short vs. long inflation of DEB in restenosis treatment in the carotid territory.
As we have shown, recurrent restenosis may be treated safely with secondary balloon angioplasty, or, in some selected cases, with balloon-mounted DES implantation. In 6 out of 8 cases of balloon-mounted DES implantation there was no further restenosis. In 2 cases, edge DES implantation resulted in further stent deformation and artery occlusion in one, as described previously [11]. This is due to the fact that the coronary DES is prone to deformation in the segment of the artery that bends and kinks. This observation prompted us to introduce self-expanding drug-eluting coronary stent for patients with recurrent edge-stent restenosis. Mechanical properties of this stent are similar to self-expanding carotid stents in terms of radial force and resistance to deformation. Edge stent restenosis may be provoked by mechanical repeating wall injury at the border between the stent edge and native vessel wall. The compliance mismatch of the stented segment and native artery wall provokes unnatural artery kinking at the end of stent while neck movement leads in consequence to repeated local intimal irritation and inflammatory response. This phenomenon may be more pronounced with closed-cell design stents (CC). In fact, the comparison of CC and open-cell design stents (OC) indicates significantly increased stiffness in the former group [16]. We hypothesized that the CC stent may have higher rate of restenosis. In fact, this correlation was not found for the whole groups of stents (CC vs. OC); it was only demonstrated for the Carotid Wallstent. This phenomenon is difficult to explain, but there might be several mechanisms responsible for it:
Carotid Wallstent bending stiffness is similar to the majority of stents in our group, but its length change during implantation is definitely the greatest (–22% vs. –5.9% for Precise, –2.4% for Xact and +0.5% for Cristallo Ideale) [16]. Furthermore, stent shortening during follow-up, as described in several case reports, may lead to partial plaque uncovering and accelerate restenosis. Gaudry et al. report 3 out of 12 restenoses associated with Carotid Wallstent shortening. Interestingly, lack of stent coverage of the common carotid artery was an independent restenosis risk factor [17]. Moreover, the Carotid Wallstent has the lowest radial force [16], reducing the chance of further stent expansion, and it was shown that at higher radial force, the stent has better chances of making the artery conform to its original shape [18]. Non-circular stent cross section may provoke flow disturbances, increase in shear stress and in consequence stimulate neointima proliferation.
Another interesting finding is that the Carotid Wallstent is the only one made of unique cobalt-chromium-iron-nickel-molybdenum alloy containing an enhanced radiopaque tantalum core, whereas all other stents from our group are made of Nitinol (nickel-titanium alloy). There are data suggesting that in some cases in-stent restenosis may be influenced by metal allergy [19], including hypersensitivity to molybdenum [20]. The Carotid Wallstent also has the smallest free cell area in the CC group, 1.1 mm2; while for Xact it is 2.7 mm2 and ≥ 4 mm2 for other stents. The higher density of metal mesh, with possible hypersensitivity to metal, may play a role in restenosis development.
The type of stent has not yet been investigated as a potential risk factor of restenosis. In most cases, this is because of a small sample of CAS and/or a single brand of stent used. The present study showed that Carotid Wallstent use strongly correlates with higher risk of restenosis. Although the Carotid Wallstent has not been shown as an in-stent restenosis risk factor so far, our findings are not isolated. Montelione et al. report 10 out of 12 symptomatic in-stent restenosis as occurred in the Carotid Wallstent [21].
It should be kept in mind that the difference in stent-dependent segmental elasticity may increase DUS velocities for CC as compared to OC stents and that may influence the restenosis rate overestimation in the former group. This was not the case in our study, as we confirmed all critical restenosis in angiography. Interestingly, in both Acculink stents that developed critical restenosis that was treated with POBA, recurrent restenosis occurred. The small size of the group did not allow for a statistical confirmation of this observation.
Severe ICA stenosis was recognized in our group of patients as a restenosis risk factor, which is consistent with observations from the study of Gaudry et al. [17]. It has been found to be associated with higher plaque burden [22]. Bilateral ICA stenosis reflects considerable atherosclerosis progression and higher disease activity. This may explain the strong influence of bilateral ICA stenosis on further in-stent restenosis. Veselka et al. found a significantly higher rate of restenosis in the group with bilateral carotid stenosis (vs. unilateral stenosis). This group was also characterized by a higher rate of follow-up death [23]. Another study showed contralateral carotid artery occlusion as a strong independent predictor of in-stent restenosis [24].
On the other hand, ISR was treated successfully with DEB angioplasty with no third reoccurrence except for the patient with Takayasu disease.
Limitations
A limitation of the study is that it is a one-center, non-randomized registry and our findings might not apply to other populations of patients. Another limitation is possible stent selection bias as the use of stent type and brand was operator-dependent. Thus, some groups of stents are represented in small numbers and may not be able to provide sufficiently strong statistical data.
Conclusions
Our findings suggest that balloon angioplasty may not be optimal for edge-stent restenosis as this procedure does not eliminate the source of the problem that is local inflammation caused by repeating edge-stent mechanical injury. We believe that implantation of a self-expanding DES may be considered in this specific clinical situation.