Introduction
Gastric cancer (GC) remains an important cancer worldwide, ranking fifth for incidence and fourth for mortality globally [1]. Surgery is still the primary treatment option for gastric cancer. Conventional D2 gastrectomy has been widely accepted as a standard procedure of surgical treatment for local advanced gastric cancer in the world, especially in East Asian countries [2]. Since Kitano reported the first laparoscopic radical gastrectomy for gastric cancer in 1994, laparoscopic radical gastrectomy has been receiving more and more attention [3]. Compared to traditional open surgery, laparoscopic surgery has unique advantages in terms of both rapid recovery and the therapeutic effect in GC patients [4]. Up to now, laparoscopic radical gastrectomy has shown satisfactory oncological results in both early and advanced gastric cancer, even in patients with gastric cancer undergoing neoadjuvant chemotherapy [5–7]. With the continuous exploration, development and maturity of laparoscopic technology in the field of surgical treatment of gastric cancer, surgical approaches have shifted from multiport surgery, extracorporeal anastomosis, and minilaparotomy incision to reduced-port or single-port surgery, intracorporeal anastomosis, and natural orifice extraction [8].
Natural orifice specimen extraction (NOSE) laparoscopic surgery is arising as a new and promising technique minimizing surgical injury, reducing postoperative pain and promoting faster recovery [9]. This procedure allowed for specimen extraction through natural orifices including the anus, vagina, or mouth without additional abdominal incisions [10]. Currently, NOSES is widely used in colon cancer [11–13]. In June 2014, the first NOSE following gastrectomy was performed [14]. Due to the questions about the necessity to avoid abdominal incisions and the psychological concerns of most surgeons, the NOSES gastrectomy was rarely reported from then on. Meanwhile, the NOSES procedure’s rectal incision increased the risk of intestinal fistula [15]. Therefore, the safety and feasibility of NOSES in gastrectomy still remain controversial.
Aim
For its flexibility and healing ability, the vagina, the unique anatomical site of the female, has a clear advantage in NOSES [16]. Thus, this study aims to compare the safety and feasibility of laparoscopic gastrectomy following a transvaginal and transumbilical specimen extraction in female patients with GC, which may offer clinical evidence for conducting NOSES in laparoscopic radical gastrectomy for GC.
Material and methods
Patients
This was a retrospective analysis of a prospectively maintained gastric cancer database. Between January 2016 and July 2021, a total of 37 consecutive female patients with gastric cancer underwent transvaginal (NOSES group) or transumbilical (TLG group) specimen extraction following total laparoscopic gastrectomy in the Department of Gastric Surgery at Liaoning Cancer Hospital. Patients who were enrolled needed to meet the following conditions: 1) preoperative examination confirmed the diagnosis of gastric carcinoma without peripheral tissue invasion and distant metastasis; 2) no operative contraindications and the patient who did not undergo the preoperative radiotherapy or chemotherapy consented to surgical treatment; 3) no vaginal stenosis, adhesion. In addition, cases of laparoscopic gastrectomy GC-NOSES from the literature online were also included in this study. A literature search of PubMed/Wanfang/CNKI were performed for all articles in English and Chinese published from 2010 to 2021. Four case reports [8, 10, 17, 18] including 14 patients were eventually screened for the supplementary analysis. Finally, a total of 26 laparoscopic gastrectomy NOSES (center: 12 patients, case reports: 14 patients, and the detailed information of the 14 patients is shown in Table I) were identified. The clinicopathological features, operative outcomes, and postoperative course of patients with NOSES were compared with 25 patients who underwent TLG. The Ethics Committee of Liaoning Cancer Hospital approved this study and written informed consent was obtained from the patients at our center.
Table I
Clinicopathological characteristics of cases included
Pathologic staging was based on the 8th edition of the Union for International Cancer Control TNM system. Early surgical complications were defined as complications occurring within 30 days after the surgery, including anastomosis-related complications (leakage, stenosis, and bleeding), intra-abdominal infection and bleeding, wound infections and hernia, lymphatic leakage, ileus, as well as nonsurgical complications including pneumonia, cardiovascular disease and thrombosis. The time from opening the posterior fornix or abdominal wall to completing the suture of the posterior fornix or abdominal wall was defined as the period of specimen extraction.
Operative technique
Excision and reconstruction
Before surgery, all patients voluntarily signed written informed consent and agreed to have laparoscopic surgery. All operations were carried out by the same skilled surgeon, who has conducted over 300 laparoscopic gastrectomy procedures. The totally laparoscopic approach for intracorporeal anastomosis without auxiliary incision was conducted in all cases. A carbon dioxide pneumoperitoneum was created through the umbilical port, with pressure maintained at 11–13 mm Hg. In this procedure, five trocars were used, and the location distributions are presented in Figure 1. The TLG group was placed in the supine position with the patients’ legs apart. A lithotomy position was used in the NOSES group during the specimen extraction. The removal of the stomach and dissection of the regional lymph nodes were the same in both groups. Depending on the location of the primary tumor in the stomach, total gastrectomy (TG), distal or proximal gastrectomy (DG/PG) was used. At least D1+ or D2 lymph node dissection was performed according to the guidelines of the Japanese Gastric Cancer Association [19]. The duodenum was transected with a linear stapler (linear cutter 60 mm; Ethicon Endo-Surgery, USA) at least 1 cm below the pylorus.
Figure 1
Schematic drawing of placement of surgical trocars. A – Location distributions of trocars in NOSES group. B – Location distributions of trocars in TLG group, the specimen was extracted through a mini-laparotomy incision 30–50 mm around the umbilicus

Following distal gastrectomy, intracorporeal Billroth I or Billroth II was performed. In the intracorporeal Billroth I reconstruction, an end-to-end gastroduodenostomy between the greater curvature of the remnant stomach and the residual end of the duodenum was performed using the absorbable suture. In the Billroth II reconstruction, a side-to-side gastrojejunostomy was performed using a 60-mm endoscopic linear stapler (Ethicon Endo-Surgery, USA), which was also used to create a side-to-side jejunojejunostomy, also called the Braun anastomosis. For the proximal gastrectomy, a double-flap technique was performed as demonstrated by Watanabe et al. [20]. For the total gastrectomy, the Roux-en-Y reconstruction was performed using a 60-mm endoscopic linear stapler (Ethicon Endo-Surgery, USA). The common entry hole for the anastomosis was closed with the absorbable sutures.
Specimen extraction
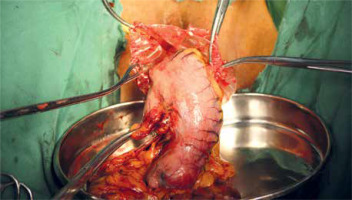
To prevent intraperitoneal tumor dissemination, the specimens were completely sealed within a large plastic bag before extraction. The specimens in the TLG group were extracted using a large plastic bag through a 3–5 cm extended C-shaped skin incision around the umbilicus. For the NOSES group, the specimens within a large plastic bag located at the upper abdomen were transferred to the pelvic cavity under laparoscopy. Before specimen removal, the pelvic cavity was confirmed to be in good condition by laparoscopy (no adhesion). The patient was placed in a lithotomy position, the posterior fornix was exposed, hemostatic water (1 : 100000 epinephrine solution) was injected, the uterine rectum depression was touched, and the curved full-layer incision was cut at a distance of 1–2 cm from the junction of the vagina and cervix, and the incision was extended to both sides of the whole layer, 5–6 cm in length. The plate-shaped press plate exposes the pelvic cavity and takes out the specimen bag. When the bag mouth reaches outside the vaginal opening, the bag mouth can be cut open according to the situation, and part of the greater omentum can be taken out, and all the specimens can be pulled homeopathically. The vaginal mucosa of the posterior fornix was sutured in full thickness in vitro. The above procedures are performed by experienced gynecologists (DBW and ZY) (Photo 1). Meanwhile, the entire procedure of transvaginal specimen extraction was carefully monitored using a laparoscope. Finally, the vaginal incision was closed extracorporeally with absorbable sutures and a check was conducted by the laparoscope in order to make sure that the vaginal incision was intact.
Postoperative management
Patient age, body mass index (BMI), and American Society of Anesthesiologists score were among the clinical data obtained from medical records. Pathologic results that were analyzed including tumor size, location, WHO classification, Lauren classification, neoadjuvant chemotherapy, number of retrieved lymph nodes, resection margins, TNM stage, Her-2 status, nerve or vascular invasion. Surgical outcomes included operation time, specimen extraction time, blood loss, time to first flatus, time to first waters, time to first fluids, time to starting a soft diet, time to removing drainage tube and postoperative hospital stay. These detailed parameter measurements are listed in the supplementary materials (Table II). The degree of postoperative pain was assessed using a visual analog scale and the number of additional days of analgesics required until hospital discharge. The total hospital cost was also analyzed.
Table II
Instructions of the evaluation outcomes
Statistical analysis
IBM SPSS Statistics version 23.0 for Windows was used for statistical analysis. Continuous data were presented as means ± SD and categorical data was presented as proportions. Categorical variables were analyzed using the χ2 test or Fisher’s exact test, whereas continuous variables were analyzed using Student’s t test. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
In total, the baseline characteristics between the two groups are shown in Table III. This research included 37 female patients. NOSE was performed on 12 patients, while TLG was performed on 25 patients. There was no significant difference in gender, age, BMI, tumor size, tumor location, operation, reconstruction, classification (WHO or Lauren), nerve or vascular invasion, and neoadjuvant chemotherapy between the two groups except for the TNM stage and Her-2 status. More stage I patients (48%) underwent TLG compared to the NOSES group.
Table III
Comparisons of clinicopathological characteristics between NOSES and TLG
[i] BMI – body mass index, ASA – American Society of Anesthesiologists score, DG – distal gastrectomy, PG – proximal gastrectomy, TG – total gastrectomy, WHO – World Health Organization, WD – well differentiated, MD – moderately differentiated, PD – poorly differentiated, NCT – neoadjuvant chemotherapy.
Surgical outcomes
All short-term surgical outcomes between the two groups are shown in Table IV. The operative time in the NOSES group was significantly shorter than in the TLG group (239.3 ±21.5 vs. 256.1 ±21.2 min, p = 0.031). During the operation in the NOSES group, the specimen extraction time through the vagina was also shorter than that through the umbilicus in the TLG group (17.0 ±4.2 vs. 30.8 ±4.3 min, < 0.01). No significant difference was observed in the comparison of the estimated blood loss between the two groups (112.5 ±69.6 vs. 120 ±64.8 ml, p = 0.749). With regard to radical validity, the number of harvested LNs in the two groups was 31.2 ±8.7 and 34.3 ±12.1 respectively (not significantly different, p = 0.443). For the comparisons of distal and proximal margin between the two groups, similar results were also found (5.0 ±4.3 vs. 6.4 ±3.1, p = 0.259; 5.7 ±1.7 vs. 5.3 ±1.5, p = 0.506). In the postoperative recovery comparisons, although there were no significant differences in the time to first flatus, the time to first waters and the time to removing the drainage tube between the two groups, the patients who underwent NOSES had a shorter time to first fluids (3.9 ±0.5 vs. 5.6 ±1.2 days, p < 0.01) and time to starting a soft diet (5.6 ±0.7 vs. 7.7 ±1.7 days, p < 0.01) compared to those underwent TLG. Postoperative pain in the NOSES group was significantly less than in the TLG group: the mean postoperative pain scores were 1.3 ±0.5 vs. 2.6 ±0.7 (p < 0.01). The patients who underwent NOSES had fewer additional days of analgesics compared to the patients who underwent TLG (0.3 ±0.5 vs. 2.0 ±1.1, p < 0.01). Postoperative hospital stay days in the NOSES group were fewer than in the TLG group (10.2 ±2.2 vs. 12.4 ±2.9 days, p = 0.030).
Table IV
Comparisons of surgical outcomes between NOSES and TLG
Postoperative complications
The complications of the two groups are summarized in Table V. The overall complication rate of the NOSES and TLG groups were 16.7% (2/12) and 24.0% (6/25), respectively. In the NOSES group, 2 patients suffered from nonsurgical complications: 1 patient experienced pneumonia and the other 1 experienced thrombosis of the deep leg veins. No surgical complications were observed in the NOSES group. In the TLG group, a total of 6 patients had complications: 4 patients experienced surgical complications and 2 patients suffered from a nonsurgical complication. In the surgical complications, 1 patient experienced anastomotic bleeding, 2 patients experienced intra-abdominal infection and 1 patient experienced lymphorrhagia. In the nonsurgical complications, 1 patient experienced pneumonia and 1 patient experienced cardiovascular disease. In the NOSES group, we also did a separate analysis of perineal damage and urinary incontinence, and it was found that there was no perineal damage or urinary incontinence in the patients who underwent NOSES. According to the Clavien-Dindo scoring system, the grading of postoperative complications from both groups were from I to II. In both groups, all patients with complications were cured and discharged after active treatment. There was no operation-related death during the perioperative period. Overall, the postoperative complications were similar in the two groups (p = 0.438).
Table V
Comparisons of postoperative complications between NOSES and TLG
Re-evaluation after case additions
Up to now, there have been very few reports about radical gastrectomy through NOSES. Moreover, these articles were in the form of case reports. Hence, we included all relevant case reports in this study by a comprehensive literature search. Four case reports [8, 10, 17, 18] including 14 patients were eventually screened and the relevant information was carefully extracted. Finally, the number of updated cases in the NOSES group was 26 and the number of cases in the TLG group did not change.
Patient characteristics
The new baseline characteristics in the two groups are shown in Table VI. There was no significant difference in most clinicopathological characteristics between the two groups except for WHO classification and neoadjuvant chemotherapy.
Table VI
Comparisons of clinicopathological characteristics between NOSES and TLG including the cases
Surgical outcomes
Although the operation time of the two groups was similar (264.8 ±66.3 vs. 256.1 ±21.2 min, p = 0.535), the results were different from our own data. In terms of the evaluations of rapid recovery including estimated blood loss, time to first flatus and postoperative hospital stay, similar results have been obtained with our own data. These results are shown in Table VII.
Table VII
Comparisons of surgical outcomes between NOSES and TLG including the cases
Postoperative complications
With the addition of 14 cases, there were still no significant differences in the incidence of surgical complications between the two groups (p = 0.439). In the new NOSES group, the two new patients suffered surgical complications: one experienced intra-abdominal bleeding and the other one experienced ileus. Similar findings were found across data analyses from our center data, as shown in Table VIII.
Table VIII
Comparisons of postoperative complications between NOSES and TLG including the cases
Settlement cost analysis
The basic hospital fee from every patients was available in our hospital information. We also analyzed the total hospital cost. The results indicated that the patients who underwent NOSES had a smaller financial burden than the patients who underwent TLG (p < 0.01, Figure 2).
Discussion
As is generally known, laparoscopic surgery aims to present a minimal abdominal wall incision and the least bowel contact during the operation. Since laparoscopy was applied to gastrectomy, its advantage of being minimally invasive gradually plays an increasingly important role in the treatment of GC [4]. Multiple randomized controlled trials (RCTs) have confirmed that the safety and long-term oncology efficacy of laparoscopic gastrectomy (LG) are not inferior to those of open gastrectomy (OG) in patients with both early (EGC) and advanced GC (AGC) [6, 21, 22]. In China, GC remains the second most common malignant tumor and the third leading cause of tumor-related deaths. It is a pity that AGC accounted for more than 80% of cases [23]. The completion of the following postoperative adjuvant chemotherapy as a conventional treatment for advanced gastric cancer was dependent on the patient’s rapid recovery after radical gastrectomy. Hence, searching for a surgical approach to facilitate the rapid recovery of both early and advanced GC patients had become the focus of gastrointestinal tumor surgeons.
In both laparoscopic-assisted gastrectomy and laparoscopic gastrectomy, the extraction of specimens was completely dependent on the abdominal incision. Natural orifice specimen extraction was a surgical method that aimed to reduce surgical trauma by removing the need for an incision into the abdominal wall [24]. The incision to extract the specimen was usually made in the rectum [13], vagina or mouth. Although the NOSES-related reports were limited in gastrectomy, the use of NOSES in both EGC and AGC had been confirmed to be feasible and safe [8, 18]. Given the posterior fornix’s elasticity and healing ability, as well as the risk of intestinal fistulas following rectal incision, a vaginal incision was typically used for specimen extraction [12]. Based on the above reasons, this study made a data comparison between transvaginal and transumbilical specimen extractions following the total laparoscopic gastrectomy in female patients with GC. Our results indicated that the patients undergoing transvaginal extraction of specimens had more advantages compared to those undergoing transumbilical extraction of specimens.
Specimen extraction is the most characteristic surgical procedure in NOSES. As observed in our study, the time of transvaginal specimen removal was significantly shorter than that of transumbilical specimen removal, which also accelerated the total operation. Due to the low flexibility of the abdominal wall itself, as well as in vitro specimens including the residual or even the total stomach, the length of the abdominal incision was uncertain. In some ways, this uncertainty also lengthens the time it takes to remove specimens and increases the probability of incisional hernia. Previous reports suggested that the incisional hernia incidence was significantly lower with the use of 5-mm trocars than with the use of larger ports and an ancillary incision in the abdominal wall [25]. Furthermore, this method of specimen extraction was designed to reduce the frequency of surgical wound discomfort and infection-related problems. Ghezzi et al. found that the size of abdominal incisions is associated with postoperative pain [26]. A 40–50 mm incision around the umbilicus significantly increased postoperative pain in patients undergoing TLG. As seen from the data of this study, patients undergoing transvaginal specimen extraction had significant advantages over those undergoing transumbilical specimen extraction in both postoperative pain scores and the use of additional postoperative painkillers. The reduction in pain also improved postoperative recovery. According to van Boekel’s findings, postoperative discomfort may be related to oral intake and bowel function. For one thing, postoperative pain could stimulate the neurohumoral stress response, including an increase in protein catabolism levels of endocrine hormones, which led to the inhibition of intestinal peristalsis [27]. For another, less pain also made patients more active out of bed, which promoted the recovery of peristaltic function. This also explained why patients undergoing NOSES had a shorter postoperative fluid diet time than the TLG group in our study. Faster postoperative recovery also reduced the cost of surgery and significantly reduced the economic burden for gastric cancer patients. This result of rapid recovery is also seen in NOSES group patients with sigmoid colon cancer or rectal cancer resection [13]. Meanwhile, similar results from faster postoperative recovery were observed when we included case reports. This suggested that in both colorectal cancer and gastric cancer, patients who had underwent NOSES had a significant advantage in terms of rapid recovery compared to transumbilical specimen extraction.
The aseptic principle and oncological safety were two major concerns in procedures of NOSES. Compared to the abdominal incision, the posterior colpotomy extended exposure time of the abdominal and pelvic cavity, causing possible contamination by vaginal microorganisms, which might increase the risk of abdomen or pelvic infection. However, both our data and the additional data from included patients showed that NOSES did not have abdominal infections. These results benefited from several factors such as our comprehensive preoperative assessments for the anatomy and physiology of the natural orifice, the administration of prophylactic antibiotics and intraoperative peritoneal irrigation. Additionally, some reports also explained that the positive pressure between the peritoneal cavity and the vagina generated by the pneumoperitoneum might prevent peritoneal bacterial contamination [28]. Importantly, in our study, all the posterior colpotomies in the NOSES group were conducted by the same experienced gynecological surgeons, which ensured professional operations and low risk of infections. The oncological safety of radical gastrectomy was critical. Before the specimens were extracted, the processes of totally laparoscopic gastrectomy, lymphadenectomy and digestive tract reconstruction were the same. There were no significant differences in tumor treatment outcomes (including retrieved lymph nodes, proximal and distal margin, and estimated blood loss) between the two groups. Transvaginal specimen extraction inevitably faced two issues: specimen transit in the abdominal cavity and specimen extraction through the vagina. The exfoliated tumor cells coming from the transport process of specimen or compression of the specimen through the narrow vagina increased the risk of recurrence. Limited to the short-term outcomes of this study, we did not analyze the long-term follow-up of the 2 patients, and the related long-term follow-up results have rarely been reported in other reports on GC. However, in colorectal cancer, long-term follow-up of the NOSES patients showed no implantation metastasis compared to the traditional umbilical specimen extraction, and there was no significant difference in survival between the two groups [13]. Certainly, larger RCT studies are needed in both colon cancer and gastric cancer. At present, the international consensus on NOSES for gastric cancer indicates that the use of a sterile plastic sleeve and pelvic irrigation could effectively prevent tumor dissemination [29]. McKenzie et al. reported in colon cancer that the risk of tumor seeding after transvaginal delivery was no higher than the risk associated with transabdominal extraction utilizing a specimen retrieval bag [30].
In comparison with transumbilical extraction, the transvaginal extraction had more potential advantages. To begin with, NOSES was better suited to extraction from larger specimens, especially in higher BMI patients compared to the TLG group. In our study, whether the cases were included in our own data or not, the BMI and tumor size of the NOSES group were larger than those of the TLG group, even though the differences were not statistically significant (BMI: 24.1 ±3.2 vs. 22.6 ±4.0, 25.1 ±4.8 vs. 22.6 ±4.0; tumor size: 4.1 ±1.5 vs. 3.8 ±2.5 cm). Studies on vaginal extraction of larger specimens have also been reported [31]. Secondly, because the position of the vagina after the fornix was very deep and there was no nerve distribution around, the injury to the posterior fornix did not lead to chronic dyspareunia or affect sexual function [32]. Also we did not find any dyspareunia problems with follow-up in the NOSES group. At the same time, no perineal injury or urinary incontinence was observed in the NOSES group. Moreover, our data indicated that NOSES was not only for menopausal females; this procedure was also performed for premenopausal patients. In the NOSES group, the youngest patient was only 37 years old and the incision hidden in the vagina could increase the cosmetic effect after surgery, which was favored by young women with gastric cancer.
In this study, some limitations must be emphasized. Primarily, patient numbers were small and the follow-up was short. Although we attempted to expand the sample size with the addition of case reports and the outcomes were stable, this also increased the possibility of heterogeneity and bias in the analysis. Next, this complicated surgical procedure required more technique and experience of the surgeons to perform the transvaginal NOSES. Thirdly, the NOSES procedure might not be suitable for patients with bulky tumors, previous pelvic surgery or radiation, or a narrow vagina. In our study, the patients who were included in the NOSES group were all strictly screened by our gynecologists before surgery. Finally, this procedure was only suitable for female GC patients and had limited application. Up to now, the reports related to NOSES have rarely been reported for GC and are mostly single centered, small-sampled, and retrospective. Poor quality and a low evidence level were their common features. Large-sampled, multicentered RCTs comparing NOSES vs transabdominal specimen extraction for GC are highly necessary.
Conclusions
Our data indicated that the patients undergoing transvaginal and transumbilical specimen extraction had the same tumor therapeutic effect. However, the patients undergoing transvaginal specimen extraction experienced a shorter operative time and shorter specimen extraction time. The procedure of transvaginal specimen extraction could significantly relieve postoperative pain and promote faster postoperative recovery, which reduced the hospital costs for patients. The patients undergoing transvaginal and transumbilical specimen extraction had a similar rates of complications. Transvaginal specimen extraction was feasible and safe for both early and advanced gastric cancer. NOSES was not only for menopausal females; this procedure was also performed for premenopausal patients.