Introduction
MicroRNAs (miRNAs) are a group of small noncoding, endogenous, single-stranded RNAs between 20 and 22 nucleotides in length that are involved in the regulation of gene expression at the post-transcriptional level [1, 2]. A characteristic feature of miRNAs is their specificity to a given tissue. They are present in various body fluids such as plasma, urine, saliva, tears and milk. Thus, miRNAs can be used as biomarkers of organ damage. In liver diseases, the primary miRNA is miR-122, which accounts for ~70% of miRNAs in liver tissue [3]. Elevated levels of circulating miR-122 have been shown to play a key role in the regulation of cholesterol and fatty acid metabolism in the liver and correlate with increased aminotransferase activity [4, 5]. In cholestatic liver diseases, overexpression of miR-21 may result from changes in the composition of the bile acid pool in the liver. Disturbed biliary homeostasis, unbalanced cell proliferation and apoptosis also result from impaired expression of miR-26b. MiR-199a plays an important role in many liver diseases, including drug-induced liver injury (DILI), metabolic dysfunction-associated fatty liver disease (MAFLD) and hepatocarcinoma (HCC). It can be used to regulate HBV replication and to assess the progression of liver fibrosis [6]. Increased expression of miRNA-34a in cirrhosis or liver steatosis raises the possibility of its use as a potential biomarker for liver cirrhosis [7, 8]. The characteristic profile of changes in miRNA expression levels can be helpful in the early diagnosis of a wide variety of conditions, including haematological, cardiovascular and hepatological conditions [9]. They can affect viral replication, modulate the antiviral response and thus influence the degree of liver damage. These molecules are stable and assayable in body fluids and therefore represent a potential source of non-invasive prognostic markers.
Epstein-Barr virus (EBV) hepatitis is usually characterised by a mild course. The possibility that severe hepatological complications may also occur indicates the need to identify predictors of liver damage. The aim of this study was to analyse the expression of miR-21-3p, miR-122-5p, miR-26b-5p, miR-34a-5p and miR-199a-5p as non-invasive biomarkers of hepatological complications among children infected with EBV.
Material and methods
This study was conducted with the approval of the Bioethics Committee at the Ludwik Rydygier Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun (Bioethics Committee Resolution No. 673/2018). The study included 68 children, with 36 girls and 32 boys, aged 1 to 18 years, hospitalised from 01.12.2018 to 31.12.2020 in the Department of Paediatrics, Infectious Diseases and Hepatology with serologically and molecularly confirmed Epstein-Barr virus infection. The study group consisted of 54 children in whom hepatological complications were observed. Within this group, two subgroups were identified: with increased alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (GGTP) activity (IA), and with patients with increased ALT activity and normal GGTP (IB). The control group (II) included EBV-infected children with normal ALT and GGTP activity. The comparative group (III) consisted of healthy children in whom the presence of EBV genetic material was excluded by molecular testing.
Alanine aminotransferase and GGTP activities were determined according to the manufacturer’s protocols using a standard enzyme-colourimetric assay (COBAS INTEGRA 400/800, Roche). Anti-EBV antibodies were detected with the LIAISON EBV IgM test (DiaSorin) using chemiluminescence immunological (CLIA) technology. The test was performed on a LIAISON XL analyser. Isolation of EBV DNA genetic material was performed using the Maxwell RSC Viral Total Nucleic Acid Purification Kit. Real-time PCR using TaqMan probes was used to quantify the EBV DNA viral load according to the manufacturer’s protocol. Sensitivity level was 16 copies/ml. Expression was studied for seven miRNAs. Of these, miR-484 and miR-16-5p were used as standardisation miRNAs. The miRNA profile analysed included 6 detectable molecules in human plasma that have a potential association with increased biochemical exponents of liver injury: miR-122-5p, miR-21-3p, miR-34a-5p, miR-26b-5p and miR-199a-5p. An expression analysis was performed based on a real-time PCR reaction using the TaqMan Fast Advanced Master Mix kit and miRNA-specific TaqMan Advanced miRNA Assay primer and probe sets.
Statistical methods
A statistical analysis and figures were performed using Microsoft’s Excel spreadsheet and IBM SPSS Statistics (version 22). The values of the parameters studied were measured as categorical and quantitative variables. The categorical variables were described with the number and percentage (%), and the quantitative variables with the median and the 25th and 75th percentiles (Q1-Q3), due to the inconsistency of the distribution of most of the analysed variables with the normal distribution. Differences in the distributions of categorical variables were assessed using Pearson’s chi-square test or Fisher’s exact test in the case of small groups. Non-parametric tests for independent groups were used to compare the quantitative variables, such as the Mann-Whitney U test for two groups or the Kruskal-Wallis test when more than two groups were being compared simultaneously. A value of p < 0.05 was used as the significance level for statistical tests.
Results
Hepatological complications were observed in a group of 54 EBV-infected children. To group IA were assigned 33 children with increased ALT and GGTP activity, while 21 patients with increased ALT activity and normal GGTP were assigned to group IB. Group II included 14 EBV-infected children without hepatological complications. Group III consisted of 13 healthy, uninfected children. For four miRNAs, significantly higher expression was confirmed in the group of EBV-infected children, i.e. for miR-21-3p, p = 0.011 (2-fold); miR-122-5p, p = 0.015 (14-fold); miR-26b-5p, p = 0.02 (1.5-fold); miR-34a-5p, p = 0.036 (9-fold). For miR-199a-5p there was no statistically significant difference between the groups (p = 0.089) (Table 1).
Table 1
Analysis of expression of selected miRNAs in the group of EBVinfected children and in the group of children not infected with EBV
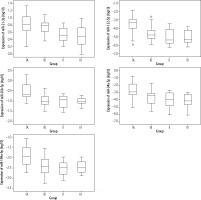
The expression of miR-122-5p, miR-26b-5p, miR-34a-5p and miR-199a-5p was the highest in group IA. Equal expression of miR-21-3p was demonstrated in groups IA and IB. The lowest expression for miR-26b-5p was observed in group IB, and for the other analysed miRNAs in group II (Fig. 1).
Fig. 1
Expression of selected miRNAs for the different groups analysed; IA – group with increased ALT and GGTP activity, IB – group with increased ALT and normal GGTP activity, II – control group with normal ALT and GGTP activity, III – comparative uninfected group
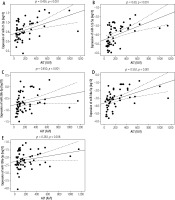
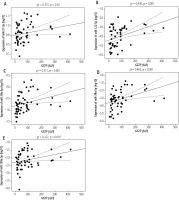
The median expression levels of selected miRNAs in EBV-infected children with and without hepatological complications are shown in Table 2. There was significantly increased expression of miR-21-3p, miR-122-5p, miR-26b-5p, miR-34a-5p, miR-199a-5p in group IA patients compared to group II (p = 0.002, p < 0.001, p < 0.001, p = 0.003, p < 0.001, respectively). In the group of 54 children diagnosed with hepatitis, statistically significant positive correlations were found between the analysed miRNAs and ALT and GGTP activity: miR-21-3p (r = 0.436, p < 0.001; r = 0.315, p = 0.02), miR-122-5p (ρ = 0.6, p < 0.001; r = 0.43, p = 0.001), miR-26b-5p (ρ = 0.45, p < 0.001; r = 0.511, p < 0.001), miR-34a (ρ = 0.592, p < 0.001; r = 0.463, p < 0.001), miR-199a-5p (ρ = 0.283, p = 0.038; r = 0.327, p = 0.016) (Figs. 2, 3). With the increase in ALT and GGTP activity, an increase in miR-21-3p, miR-122-5p, miR-26b-5p, miR-34a-5p, miR-199a-5p expression was observed.
Table 2
Median values (Q1-Q3) of expression of selected miRNAs infected with EBV with hepatological complications (IA, IB) and from the control group (II)
Discussion
To date, more than 700 miRNAs have been identified in humans. The characteristic profile of changes in miRNA expression levels can be helpful in the early diagnosis of a wide variety of ailments including haematological, cardiovascular and hepatological conditions [9]. They can affect viral replication, modulate the antiviral response and thus influence the degree of liver damage. These molecules are stable and assayable in body fluids and therefore represent a potential source of non-invasive prognostic markers. Epstein-Barr virus is an oncogenic virus with the capacity for latency. It encodes miRNAs targeting both viral and host genes involved in the immune response. Downregulation of expression of miRNAs encoded by EBV increases the response of CD8+ and CD4+ cytotoxic T lymphocytes in infected host cells. In addition, EBV is able to deregulate the expression of various host miRNAs that control immune-related processes and signalling pathways associated with the response to infection. In our own study, in a group of 54 EBV-infected children with hepatological complications, a positive correlation was found between the expression of miR-122 and the activity of ALT and GGTP. This is consistent with the results obtained by Roderburg et al., who found a significant increase in miR-122 in the serum of critically ill patients with liver damage [10]. Increased expression of miR-122 is more specific to liver disease due to synthesis mostly taking place in the liver. Therefore, the results suggest the possibility of using miR-122 expression as a liver injury-specific predictor.
In our study, a positive correlation between miR-21 expression and ALT and GGTP activity was observed in children with hepatological complications in the course of EBV infection.
In the group of EBV-infected patients with hepatitis and biliary pole injury, miR-21 expression was statistically significantly higher than in the EBV-infected group without hepatological complications. The obtained results may suggest the possibility of using miR-21 as a risk factor for the development of hepatological complications accompanying EBV infection.
There is evidence that miRNA-34a-5p may serve as a potential biomarker for cirrhosis, as its expression is elevated in the serum of cirrhotic patients and significantly correlates with aspartate aminotransferase (AST) and ALT activity [8]. These findings may suggest the usefulness of miR-34a as a prognostic factor for progression to cirrhosis and development of HCC. In our study, we observed statistically significantly higher expression of miR-34a in a group of EBV-infected children compared to a group of healthy children. In the group of children with hepatitis and biliary pole damage, miR-34a expression was significantly elevated compared to EBV-infected children without hepatological complications. In addition, a positive correlation between ALT and GGTP activity and miR-34a expression was obtained in all 54 patients with hepatological complications. The obtained findings are consistent with those presented in Farag et al.’s study of an analysis of 34 patients with histopathologically confirmed hepatic steatosis and 28 patients with non-alcoholic steatohepatitis. The expression of the analysed miRNAs was significantly higher in the analysed groups compared to healthy individuals. A statistically significant correlation was observed between AST and ALT activity and the expression of miR-34a and miR-122, and a negative correlation with albumin concentration and platelet count. In addition, a significant positive correlation was found between C-reactive protein (CRP) levels and the activity of aminotransferases, GGTP and the expression of miR-122 and miR-34a [11]. The study by Farag et al. suggests significant predictive value and specificity of miR-34a in the diagnosis of hepatic steatosis, especially when combined with CRP concentration analysis and miR-122 expression, making it a prognostic panel with higher specificity than fibrosis-4 (FIB-4), offering the possibility of avoiding liver biopsy. The use of these markers in routine clinical practice would enhance the ability to assess liver fibrosis in the outpatient setting at the primary care level.
In our study, miR-26b expression was 1.5-fold higher in EBV-infected children compared to healthy individuals. In the group of patients with hepatological complications, a positive correlation between miR-26b expression and ALT and GGTP activity was found, which may suggest an effect of increased miR-26b expression on the development of hepatological complications in EBV-infected children.
Murakami et al. as well as Messner et al. in their analyses showed that miR-199a overexpression was significantly associated with liver fibrosis progression and can be used as a non-invasive biomarker of liver fibrosis progression [12, 13]. Zhang et al. in their study confirmed a statistically significant increase in miR-199a expression in NASH [14]. Balasubramaniyan et al. were the first to demonstrate the effect of miR-199a-5p on ABCB11/Abcb11 expression in their analysis. They also confirmed that miR-199a expression is increased in cholestatic liver disorders [15]. Dai et al. in their study directly analysed the effect of miRNAs on endoplasmic reticulum (ER) stress genes in hepatocytes. The study revealed a direct link between miRNAs and hepatic ER stress and showed that miR-199a-5p suppressed prolonged ER stress, thereby preventing hepatocyte apoptosis [16]. Our study showed that miR-199a expression in EBV-infected children with hepatological complications significantly correlates with ALT and GGTP activity. The correlation obtained in this study confirms the importance of miR-199 expression in the maintenance of liver homeostasis and its potential to be used as a non-invasive biomarker of hepatocyte damage and, in particular, of concomitant cholestatic complications.
The limitation of the study is the small size of the analysed group. The confirmation of the results of this publication will require further research on a larger number of patients.