Introduction
Spitz nevi (SN) include a wide range of benign nevi first described by Sophie Spitz in 1948 [1, 2]. SN are diagnostically ambiguous due to their clinical similarity to melanoma (MM). Dermoscopy has contributed significantly to improving the clinical diagnosis of pigmented and non-pigmented SN. More recently, reflectance Confocal Microscopy (RCM) has contributed substantially to improving a differential diagnosis between SN and melanoma [3]. SN occurs mainly in children and adolescents. MM is more frequent in the middle age and past the age of forty than among younger patients [1, 2, 4]. The dermoscopic patterns most commonly associated with SN are starburst, negative network, non-specific and homogenous. Johr and Stolz described in total six dermoscopic patterns of SN. In addition to the four patterns mentioned above, they also included lesions with black pigment network and pink lesions [5–7].
In some cases, their presentation overlaps with the clinical features of malignant melanoma. The differential diagnosis of such lesions can be challenging. However, histopathological evaluation of SN reveals distinct patterns of genetic aberrations compared to melanoma and common nevi [7–9].
Many attempts have been made to determine dermoscopic diagnostic criteria of SN [10, 11]. RCM is a promising and useful tool in differentiating SN from MM and other melanocytic proliferations.
A non-invasive approach could open the possibility of a more precise pre-histological diagnosis with the reduction of surgical excision of SN.
Aim
This study aimed to describe dermoscopic and RCM features of SN compared to MM and assess the RCM usefulness in the final differential diagnosis.
Material and methods
A retrospective study including 26 melanocytic lesions was performed in three dermatologic centres (Private Dermatology Clinic in Tychy, Poland, between January 2016 and December 2020, Department of Dermatology at the University of Trieste, Italy from October 2017 to January 2020 and Military Institute of Medicine in Warsaw, Department of Dermatology, between January 2018 and January 2020).
Patients with a histopathological diagnosis of SN and MM who underwent RCM and dermoscopic evaluation were included in the study. Four SN regarding children under 12 years old were clearly benign in dermoscopy, with no biopsy, since the lesions were stable clinically and dermoscopically.
All images were analysed by certified dermatologists. The dermoscopic diagnosis was performed using standard terminology to qualify four dermoscopic patterns of SN: starburst, negative network, and homogenous or non-specific pattern. Dermoscopic images were analysed by A.P.A., C.C., and E.F. Confocal imaging was performed with a reflectance confocal microscope in vivo (Vivascope1500; Lucid Inc., Rochester, NY, USA). For each lesion, a minimum of three mosaics were available for re-evaluation: one at the level of the superficial epidermis (stratum granulosum/spinosum), one at the level of the dermo-epidermal junction (DEJ), and one from the upper dermis.
RCM features assessed at the epidermis level and DEJ are reported in Table 1. The RCM examinations blinded to histology have been re-evaluated by two experts (A.P.A. and M.S.).
Table 1
Confocal features of the epidermis and dermo-epidermal junction in Spitz nevus and melanoma
The study was conducted in compliance with the Declaration of Helsinki. For each evaluation, written informed consent was obtained.
Statistical analysis
To assess the differences in the presence of a given feature between patients diagnosed with MM or SN, a proportion test was performed for two independent trials. Statistica 13.1 software (StatSoft Polska, Krakow, Poland) was used for all statistical analyses. Values were considered statistically significant at p < 0.05.
Results
Study group characteristics
A total of 26 lesions (15 SN and 11 MM) were included. The mean age of the participants was 24.3 years (range: 5–43) in the SN group and 51.9 years (range: 36–73) in the MM group. The mean thickness of MM was 0.43. Two MM were in situ; all were superficial. Three MM were located on the limbs, while 8 on the trunk. Eight SN were located on the trunk and 7 on the skin of limbs.
Dermoscopic features
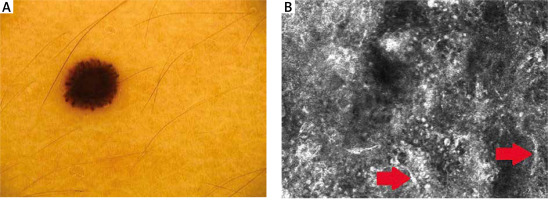
Dermoscopic data are presented in detail in Table 2. Thirteen Spitz nevi showed a “starburst” pattern (Figure 1 A). One was homogenous, and one had a negative network. Eleven MM dermoscopically revealed atypical globules. Polymorphic vessels, atypical network, and blue-whitish veil were observed in 7 melanomas. Asymmetry was clearly marked in 8 MM. Asymmetry, whitish veil, atypical globules, atypical network, polymorphic vessels, and the presence of more than 1 colour were significantly more prevalent in melanomas than in Spitz nevi (Table 2).
RCM features
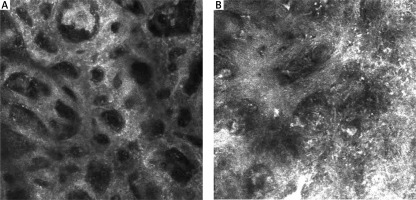
RCM features of the epidermis are presented in Table 1. Nine RCM features at the level of the epidermis were assessed. A typical honeycomb pattern was observed in 6 SN with statistical significance (p < 0.05), while 7 revealed an atypical honeycomb pattern (Figure 2 A, B). Six SN also partially revealed a cobblestone pattern due to higher pigmentation of lesions. 9 SN presented pagetoid cells in the epidermis (Figure 1 B). A significant feature of the epidermis in melanoma cases was atypical infiltration and disarray of the epidermis (p < 0.05).
Figure 2
A – A typical honeycomb pattern in SN. B – An atypical honeycomb pattern with numerous atypical cells in MM
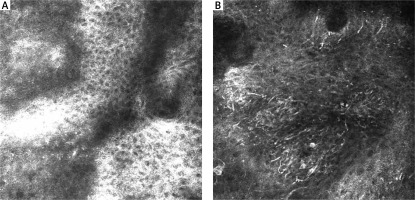
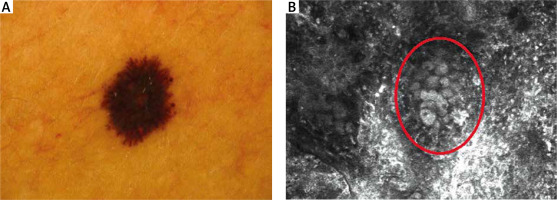
RCM features of the DEJ are presented in Table 1. At the DEJ, six features were statistically significant. SN mostly revealed the meshwork pattern at the DEJ (Figure 3 A). In our study, 11 SN showed a meshwork pattern with statistical significance (p < 0.05), while 10 MM revealed an atypical meshwork pattern with statistical significance (p < 0.05) (Figure 3 B). Atypical pagetoid cells were visible in 5 MM (p < 0.05). Cerebriform nests and sheet-like structures, essential features of malignancy, were observed in 9 MM (p < 0.05) (Figure 4 B).
Discussion
SN are benign melanocytic lesions mostly identified by characteristic clinical and dermoscopic features. Although histopathological and dermoscopic criteria of SN have been considerably described, differential diagnosis of SN and MM is challenging [12–14]. In our study, most SN and MM in dermoscopy presented a “starburst” type of architecture (Figure 4 A). Dermoscopic features differed between MM and SN, with six of them reaching statistical significance. In symmetric and well-demarcated cases, differential diagnosis with melanoma is easier. The difficulties may occur in lesions with non-specific dermoscopic patterns [15–18]. Especially when blue colour within the lesion is observed. Pellacani et al. [19] described the most common features of SN in RCM. There were cut-off, dense junctional, and plump, bright cell nests. A broadened honeycomb pattern of the epidermis, disarranged epidermal pattern, and the presence of small pagetoid cells were more likely correlated with a melanoma diagnosis. Non-edged papillae were significant for MM diagnosis, while dense junctional nests were more common in SN [12, 18]. Previous studies showed different RCM epidermal features between MM and SN; a correlation between striking cells pleomorphism and melanoma was found [20]. Carrera and Marghoob [21] highlighted the importance of the distribution of epidermal pagetoid cells within SN. In SN, pagetoid cells are rare, distributed in the suprabasal layers, and mostly clustered toward the lesion centre. In melanoma, pagetoid cells are located randomly in a rather diffuse manner. However, both MM and SN can manifest architectural disarray and cytologic atypia. In our study, there were significant differences in the architecture of the epidermis between melanoma and Spitz nevi. MM showed an atypical honeycomb pattern, while most SN revealed typical epidermal architecture. The DEJ also showed significant differences between MM and SN. Most SN revealed a meshwork pattern, whereas melanoma had an atypical meshwork pattern. Cerebriform nests and sheet-like structures proved to be essential diagnostic features as they may have a significant role in melanoma recognition. It is worth mentioning that no study to date has shown the relevance of sheet-like structure presence in the differential diagnosis between MM and SN in RCM. This significant feature presents as clusters of atypical cells in the histopathological evaluation.
Conclusions
A combined approach using dermoscopy and RCM improves the differential diagnosis of SN and MM. The main limitation of our study was a small group of patients. Although our study showed some significant differences between SN and MM in RCM, further research on a larger group should be considered.