Introduction
Malignant hilar obstruction (MHO) cases comprise 58–75% of all instances of malignant extra-hepatic biliary obstruction [1, 2]. The prognosis of MHO patients is generally poor, with a 5-year overall survival (OS) rate of less than 10% [3–5]. As patients suffering from MHO incidence are often diagnosed when the underlying disease has already reached an advanced stage, they are generally ineligible for surgical resection [6–8].
Palliative care options for MHO patients generally entail biliary stent insertion, which can rapidly alleviate jaundice and provide patients with the opportunity to undergo subsequent antitumour treatment [6–8]. Prior meta-analyses have suggested that bilateral stenting can achieve superior clinical success rates to those associated with unilateral stenting when treating MHO patients, while also lowering rates of stent dysfunction [9, 10]. Different stent insertion strategies in MHO patients can additionally determine the clinical efficacy of stent insertion [11–21]. Endoscopic and percutaneous approaches are the most common strategies employed for biliary stent insertion [11–21]. Some prior meta-analyses have examined the relative clinical efficacy of percutaneous and endoscopic biliary drainage strategies in MHO patients [22–24]. No meta-analyses to date, however, have sought to compare clinical outcomes in MHO patients undergoing percutaneous trans-hepatic biliary stenting (PTBS) or endoscopic biliary stenting (EBS).
Aim
This meta-analysis was designed to evaluate the relative efficacy of PTBS and EBS in MHO patients.
Material and methods
Study design
The Preferred Reporting Items for Systematic review and Meta-Analysis checklist was used to guide this meta-analysis [25], which was registered at INPLASY.COM (No. INPLASY2022110156).
All relevant studies published as of November 2022 were identified by searching the PubMed, Web of Science, and Wanfang databases as follows: ((((percutaneous) AND (((endoscope) OR (endoscopic)) OR (endoscopy))) AND (((biliary obstruction) OR (biliary stenosis)) OR (cholangiocarcinoma))) AND (hilar)) AND ((stent) OR (drainage)).
Eligible studies for inclusion were as follows:
types of studies: comparative studies;
diseases: MHO patients;
types of interventions: PTBS vs. EBS;
languages: no limitations.
Excluded studies included the following:
Analyses of study quality
The Cochrane risk-of-bias tool was used to assess randomized controlled trial (RCT) quality [26], whereas the Newcastle-Ottawa scale (NOS) was used to assess retrospective studies [27].
Data extraction
Data were independently extracted from studies by 2 authors, including baseline data (first author, year of publication, country, quality assessment), patient data (number of patients, age, gender, Bismuth types, tumour types, tumour stages, stent types), and treatment data (stenting technical success rates, stenting clinical success rates, stenting-related complications, stent patency, OS).
Definitions
Technical success was defined as the successful deployment of the inserted stent across the site of the obstruction [15]. Clinical success was defined as a reduction in total bilirubin level to < 75% of the baseline level prior to treatment within a 1-month follow-up period [14]. Major complications associated with stenting included haemorrhage, cholangitis, and pancreatitis. Stent patency was the interval between stenting and jaundice recurrence [16], while OS was the interval between stenting and all-cause death.
Statistical analysis
RevMan 5.3 was used to pool data related to study outcomes. Categorical variables were compared based on pooled odds ratios (ORs) and 95% confidence intervals (CIs), while OS and stent patency were analysed based on the log hazard ratio (HR) and SE. Heterogeneity was analysed based on the I2 statistic and the Q test, with random-effects models being used in cases of significant heterogeneity (I2 > 50%), while fixed-effects models were used in other cases. A leave-one-out sensitivity analysis approach was employed when seeking to define potential sources of heterogeneity. Egger’s test was used to probe for possible publication bias using Stata 12.0. P < 0.05 was defined as the threshold for statistical significance.
Results
Study selection
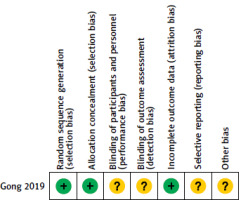
The initial literature search retrieved 650 articles, of which 11 were ultimately included in the final meta-analysis (Figure 1), including one RCT [11] and 10 retrospective studies [12–21]. Figure 2 depicts the risk of bias results for this RCT, while all 10 retrospective studies exhibited NOS scores ranging from 6 to 8. For baseline data pertaining to these 11 studies (Table I).
Table I
Baseline data of the included studies
| First author | Publication year | Country | Study design | Newcastle-Ottawa Scale |
|---|---|---|---|---|
| Gong [11] | 2019 | China | Randomized controlled trial | – |
| Huang [12] | 2016 | China | Retrospective | 7 |
| Jang [13] | 2017 | South Korea | Retrospective | 8 |
| Liang [14] | 2021 | China | Retrospective | 8 |
| Lubbe [15] | 2022 | South Africa | Retrospective | 8 |
| Paik [16] | 2009 | South Korea | Retrospective | 8 |
| Shi [17] | 2012 | China | Retrospective | 8 |
| Wang [18] | 2017 | China | Retrospective | 7 |
| Yang [19] | 2010 | China | Retrospective | 6 |
| Yang [20] | 2021 | China | Retrospective | 8 |
| Zhu [21] | 2020 | China | Retrospective | 8 |
These 11 studies enrolled 530 and 645 MHO patients who underwent PTBS and EBS, respectively (Table II). Patient baseline data are summarized in Table II.
Table II
Baseline data of the patients in the included studies
| Study | Group | Number | Male/female | Age [years] | Bismuth type | Tumour type | Tumour stage | Stent type | Stent insertion |
|---|---|---|---|---|---|---|---|---|---|
| Gong [11] | PTBS | 48 | 26/22 | 59.3 | I–IV | C | NG | Metal, plastic | NG |
| EBS | 47 | 25/22 | 60.6 | I–IV | C | NG | NG | ||
| Huang [12] | PTBS | 62 | 39/23 | 58.8 | NG | C | NG | Metal | NG |
| EBS | 69 | 42/26 | 58.7 | NG | C | NG | NG | ||
| Jang [13] | PTBS | 41 | 25/16 | 66.3 | III/IV | multiple | NG | Metal | Unilateral, bilateral |
| EBS | 69 | 46/23 | 71 | III/IV | multiple | NG | |||
| Liang [14] | PTBS | 48 | 29/19 | 58 | III/IV | multiple | I-IV | Metal, plastic | Unilateral, bilateral |
| EBS | 97 | 53/44 | 62 | III/IV | multiple | I-IV | |||
| Lubbe [15] | PTBS | 140 | 60/80 | 58.7 | I–IV | multiple | NG | Metal, plastic | Unilateral, bilateral |
| EBS | 153 | 63/90 | 61.8 | I–IV | multiple | NG | |||
| Paik [16] | PTBS | 41 | 32/9 | </≥ 65: 18/23 | III/IV | C | NG | Metal | Unilateral, bilateral |
| EBS | 44 | 26/18 | </≥ 65: 20/24 | III/IV | C | NG | |||
| Shi [17] | PTBS | 31 | 23/8 | 54.8 | II–IV | C | NG | Metal | Unilateral, bilateral |
| EBS | 44 | 29/15 | 55.7 | II–IV | C | NG | |||
| Wang [18] | PTBS | 30 | 30/25 for all | 60.2 for all | NG | multiple | NG | Metal | Bilateral |
| EBS | 25 | NG | multiple | NG | |||||
| Yang [19] | PTBS | 11 | 44/34 for all | 63 for all | NG | multiple | NG | Metal, plastic | NG |
| EBS | 6 | NG | multiple | NG | NG | ||||
| Yang [20] | PTBS | 38 | 25/13 | 70.1 | III/IV | C | NG | Metal, plastic | Unilateral, bilateral |
| EBS | 49 | 33/16 | 69.3 | III/IV | C | NG | |||
| Zhu [21] | PTBS | 40 | 22/18 | 70.8 | I-IV | C | NG | Metal | Unilateral, bilateral |
| EBS | 42 | 24/18 | 68.1 | I-IV | C | NG |
Technical success
Technical success rates were reported in 6 studies [11–13, 15, 17, 19], revealing a significantly higher pooled technical success rate in the PTBS group as compared to the EBS group (87.8% vs. 76.3%; OR = 2.41; p < 0.0001, Figure 3 A). No significant heterogeneity was detected (I2 = 48%), nor was there any evidence of publication bias (Egger’s test: p = 0.681).
Clinical success
Clinical success rates were reported in 6 studies [13–16, 18, 19], revealing a comparable pooled clinical success rate in both groups (79.0% vs. 70.9%; OR = 1.26; p = 0.45, Figure 3 B). Significant heterogeneity was detected (I2 = 51%) and was found to be attributable to the study performed by Lubbe et al. [15] in sensitivity analyses. There was no evidence of publication bias (Egger’s test: p = 0.718).
Cholangitis
Cholangitis rates were reported in 8 studies [12–17, 20, 21], revealing significantly lower pooled cholangitis rates in the PTBS group as compared to the EBS group (17.2% vs. 24.6%; OR = 0.51; p = 0.03, Figure 3 C). Significant heterogeneity was detected (I2 = 59%) and was found to be attributable to the study performed by Lubbe et al. [15]. There was no evidence of publication bias (Egger’s test: p = 0.177).
Haemorrhage
Haemorrhage rates were reported in 7 studies [11–14, 16, 17, 20], and pooled analyses revealed these rates to be comparable in both the PTBS and EBS groups (5.6% vs. 4.6%; OR = 1.53; p = 0.57, Figure 3 D). Significant heterogeneity was detected (I2 = 59%) and was found to be attributable to the study performed by Huang et al. [12]. There was no evidence of publication bias (Egger’s test: p = 0.926).
Pancreatitis
Pancreatitis rates were reported in 8 studies [11–13, 15–17, 20, 21], and pooled analyses revealed significantly lower pancreatitis rates in the PTBS group as compared to the EBS group (1.6% vs. 8.4%; OR = 0.25; p < 0.0001, Figure 3 E). No significant heterogeneity was detected (I2 = 26%). There was no evidence of publication bias (Egger’s test: p = 0.873).
Stent patency
It was possible to extract logHR and SE values corresponding to stent patency from 3 studies [13, 16, 20]. Pooled analyses indicated that patency was comparable in both groups (HR = 1.00; p = 0.96, Figure 3 F). Significant heterogeneity was detected (I2 = 59%) and was found to be attributable to the study performed by Paik et al. [16]. There was no evidence of publication bias.
OS
It was possible to extract logHR and SE values corresponding to patient OS from 4 studies [13, 14, 17, 20]. Pooled analyses indicated that OS was comparable in both groups (HR = 0.99; p = 0.73, Figure 3 G). While significant heterogeneity was detected (I2 = 63%), sensitivity analyses failed to detect the source of such heterogeneity. There was no evidence of publication bias.
Subgroup analyses
Subgroup analyses were performed focused on patients with Bismuth type III/IV MHO (Table III), but no significant differences were observed with respect to any of the analysed study endpoints when comparing these 2 patient groups.
Table III
Subgroup analyses based on the Bismuth type III/IV patients
Subgroup analyses were also performed for hilar cholangiocarcinoma patients (Table IV). In this subgroup, significantly higher pooled technical success rates were observed in the PTBS group relative to the EBS group (p = 0.01), whereas the pooled cholangitis and pancreatitis rates in the PTBS group were significantly lower than in the EBS group (p = 0.0004 and 0.007, respectively).
Table IV
Subgroup analyses based on the hilar cholangiocarcinoma patients
Discussion
The present meta-analysis was designed to compare the relative clinical efficacy of PTBS and EBS treatments for MHO patients. A previous meta-analysis compared the clinical outcomes between percutaneous and endoscopic biliary catheter drainage for MHO patients [23]. In contrast, this present meta-analysis only focused on the use of percutaneous and endoscopic stent insertion for MHO patients.
PTBS was associated with significantly improved technical success rates as compared to EBS, potentially because this strategy enables precise lobar selection [22]. In contrast, endoscopic biliary drainage has only one retrograde direction, and manipulating devices through this long channel can be challenging, indicating that PTBS may be a more appropriate treatment option for many MHO patients. PTBS is particularly important in patients in whom EBS fails as a consequence of congenital, post-surgical, or traumatic alterations to the associated anatomy [28].
Despite the superior technical performance, PTBS was not found to be superior to EBS with respect to clinical success rates in this meta-analysis, in line with prior reports comparing percutaneous and endoscopic biliary drainage strategies in MHO patients [23, 29]. The efficacy of stent drainage may thus not be impacted by the stenting approach initially employed. Indeed, the efficacy of stent insertion is primarily associated with the liver drainage area [10], with bilateral stenting generally being the most appropriate option for MHO patients [10].
Major clinical complications that can occur in patients undergoing biliary stenting include haemorrhage, cholangitis, and pancreatitis. In this study, patients who underwent PTBS exhibited lower pooled cholangitis rates. This may be attributable to the relatively aseptic nature of the PTBS procedure relative to the EBS procedure, thus decreasing the risk of bacterial introduction into the biliary tract [21]. Temporary catheter drainage was also routinely retained following stent placement, increasing external biliary drainage and thereby facilitating the more rapid discharge of contrast, further decreasing cholangitis incidence [21].
The PTBS approach was also herein found to be associated with a lower pancreatitis risk as compared to the EBS approach in MHO patients. When placed via the PTBS approach, stents usually do not cross over the ampulla. In contrast, the EBS approach necessitates the crossing of the ampulla, significantly elevating pancreatitis risk rates. Low pooled haemorrhage rates were observed in this study, and these rates were similar in both groups. This is consistent with the fact that haemorrhage is a less common complication of stenting in MHO patients as compared to pancreatitis or cholangitis.
No differences in stent patency or OS were observed when comparing the PTBS and EBS approaches in pooled analyses. Previous research has shown that bilateral stenting can prolong stent patency [10, 20], providing 2 drainage routes such that one can still facilitate drainage even when the other is re-obstructed [20]. The most effective means of improving patient OS is the administration of appropriate postoperative anti-cancer treatments [30].
In an initial subgroup analysis, similar clinical efficacy and safety outcomes were observed in Bismuth type III/IV patients. Only 2 and 3 studies reported technical success and pancreatitis rates, respectively, in this subgroup analysis, thus reducing the overall sample size. While the difference in cholangitis was not significant in this analysis, there was a clear trend towards lower cholangitis rates in the PTBS group relative to the EBS group (p = 0.06).
A second subgroup analysis suggested that PTBS exhibited advantages over EBS with respect to technical success and complication rates when treating hilar cholangiocarcinoma patients. This suggests that the disease type underlying the MHO diagnosis is not ultimately associated with the clinical efficacy of the PTBS or EBS approaches.
There are some limitations to this meta-analysis. Firstly, it only included a single RCT, and all other studies were retrospective analyses, thus potentially contributing to some level of bias with respect to these results. Secondly, roughly half of the included articles enrolled patients with multiple forms of cancer, potentially introducing additional bias. Thirdly, tumour stages were only reported in a single study [14], and stage-based subgroup analyses were thus not possible. Finally, the majority of these studies were performed in Asia, and future efforts should be made to incorporate data from other sources throughout the globe.