Heat urticaria (HU) is a rare and difficult-to-treat form of chronic inducible urticaria (CIndU) which develops locally in sites of heat application [1]. HU wheals are usually limited to the area of heat exposure; they typically develop within a few minutes after heat contact and resolve after 1 to 3 h. To reproduce lesions, a challenge test should be performed with metal/glass cylinders, filled with water, a hot water bath, TempTest® [2]. In patients with a positive response, temperature and/or stimulation time thresholds should be assessed [3]. Non-sedating H1 antihistamine drugs have been observed to be effective against HU and other types of inducible urticaria and are generally used as the first-line symptomatic therapy [4]. Omalizumab, a recombinant humanized anti-IgE antibody, has been long studied and approved as a second-line treatment for chronic spontaneous urticaria (CSU) [5]. Its efficacy observed in patients with CSU has led physicians to prescribe omalizumab as an off-label therapy for patients with different types of antihistamine-resistant CIndU, showing substantial benefits. The British Association of Dermatologists, in their latest guidelines for the management of patients with chronic urticaria, reviewed the literature available regarding the treatment of CIndU patients unresponsive to antihistamine therapy, and introduced a recommendation for the use of omalizumab as a second-line treatment for people with all types of inducible urticaria whose symptoms are inadequately controlled by first-line options [4]. Still, data regarding its efficacy and safety in CIndU patients are lacking (especially in the case of HU) and in Italy omalizumab is currently indicated only for CSU patients (www.gazzettaufficiale.it/eli/id/2021/09/30/21A05680/sg).
In this report we describe 2 cases of patients with refractory HU treated with omalizumab.
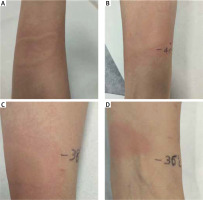
Case 1: A 56-year-old woman presented to our referral centre in July 2017. Fifteen years earlier, she had developed the first episode of heat urticaria. After this episode, she reported frequent episodes of urticaria after exposure to hot water, the hairdryer, radiator and when she had fever. Before referral, the patient had been treated with bilastine and desloratadine without success. A treatment course with levocetirizine was prescribed (10 mg b.i.d., for 4 weeks). Despite this treatment, the patient continued to experience episodes of HU. We performed heat provocation testing with a glass cylinder filled with hot water at a temperature of 44°C applied to the skin of the arm for 5 min, which proved positive 10 min after the challenge testing (Figure 1 A). In fact, wheals developed after heat exposure, with spontaneous resolution after 2 h. In order to establish a stimulation threshold, we carried out tests for different temperatures of 42°C, 40°C, 38°C, which were all positive (Figures 1 B, C). The test at a temperature of 36°C proved negative (Figure 1 D). Urticaria disease activity was determined by the Urticaria Activity Score 7 (UAS7), while a validated Urticaria Control Test (UCT) was used to monitor the patient’s subjective response to treatment, as previously described [1]. The UAS7 score was 28/42 and the UCT score 2/16 points.
The patient was then given omalizumab 300 mg subcutaneously every 4 weeks for 24 weeks. She reported a significant improvement of symptoms already within 3 weeks after the first injection. Her condition continued to improve and, in week 24 after the start of omalizumab, our patient was almost entirely symptom-free. Indeed, the UAS7 and UCT scores were recorded as 2 and 10, respectively. At the last follow-up, 8 weeks after the last administration of omalizumab, the patient had experienced a relapse of the disease, with a similar intensity of symptoms to the pre-treatment period (UAS7 score = 36).
Therefore, a new omalizumab treatment was prescribed. The patient reported complete remission of HU within 2 weeks after the first injection and continued to receive 300 mg of omalizumab every 4 weeks for a total of 20 weeks, without experiencing any symptoms (UAS7 = 0). At the end of the treatment, we repeated the heat provocation and threshold test using a glass cylinder with hot water at 40°C, 42°C, 44°C and 46°C, which resulted negative at all temperatures. However, 3 months after the last dose of omalizumab, symptoms reappeared, eliciting HU.
Case 2: A 20-year-old man with HU experienced erythematous, intensely pruritic skin lesions during the last 2 years, which developed within minutes after exposure to high temperature, especially after contact with hot air or hot water. The patient did not respond to oral antihistamine treatment including fexofenadine 180 mg b.i.d. for 1 month and ebastine 20 mg t.i.d. for 2 months. We provoked HU symptoms in the patient using a glass cylinder filled with hot water at a temperature of 44°C applied to the skin of the arm for 5 min, which proved positive 10 min after challenge testing. The patient was administered a dose of 300 mg/month of omalizumab for 3 months. After his first injection, he achieved complete remission. However, 2 months after the last anti-IgE dose, the patient experienced relapse of the disease. Omalizumab was then reintroduced with a good effect and 13 months later, the patient continues to be symptom-free on omalizumab 300 mg given every 4 weeks.
The pathogenesis of HU is not fully understood but is thought to involve histamine release from mast cells [3]. Omalizumab binds free serum IgE and prevents its attachment to the high-affinity receptor on mast cells and basophils, resulting in a decreased histamine release from these cells [6].
The efficacy of anti-IgE treatment has been shown in many disorders with a complex and unclear aetiology, including CSU, CIndU, and angioedema [1, 6, 7]. A subgroup of patients with CSU exhibits IgE autoantibodies against autoantigens, such as thyroperoxidase or double-stranded DNA, which might chronically activate mast cells and basophils. Passive transfer experiments have indicated the existence of a mast cell-activating IgE serum factor in some patients with symptomatic dermographism, solar urticaria and cold urticaria [8]. A pathogenic role has been hypothesized for IgE in cold-induced mast cell activation in patients with cold urticaria [9]. Omalizumab, in the HU of our patient, may work by reducing mast cell activation, reversing basopenia, improving basophil IgE receptor function, reducing the activity of IgG autoantibodies against FcεRI and IgEs and reducing IgE autoantibodies against an antigen or autoantigen, decreasing coagulation abnormalities associated with disease activity [10, 11]. Studies of additional patients will be necessary to understand the role of IgE in the pathogenic mechanism of the disease.
In conclusion, these two cases show that HU unresponsive to antihistamine treatment can be rapidly and successfully treated with omalizumab without side effects. Omalizumab could be a valid, safe and effective therapeutic option for patients suffering from HU who do not respond to conventional treatment.