Introduction
Traumatic diaphragmatic rupture (TDR) is a relatively common injury occurring as a result a blunt or penetrating injury to the chest [1]. Incidences of 0.8–7% and 15% have been reported following blunt and penetrating traumas respectively [2]. However, it is likely to be higher since diagnosis is said to be missed in about 60% of patients with multiple injuries and even in patients who may have undergone laparotomy for other associated injuries [2, 3]. A prevalence of 6.5% was reported by Adegboye et al. upon review of 1778 patients with thoracic trauma [4]. It is most commonly a consequence of an impact to the abdomen following road traffic accidents [5]. It may also be seen following stab or gunshot injuries to the chest or abdomen [6]. It has been reported to have a male predilection and occurs commonly in the third decade of life [3].
It was first reported in 1541 by Sennertus, who discovered a diaphragmatic defect following a gunshot injury while performing an autopsy on a patient who had died 8 months after the gunshot injury of herniation and strangulation of the colon through that defect. The first ever successful diaphragmatic repair was performed in 1886 by Riolfi in a patient who had developed a prolapse of the omentum [7, 8]. Several retrospective studies have shown occurrence of TDR to be common among young male adults in the third and fourth decades of life [6, 9–11]. The earliest review on traumatic diaphragmatic ruptures was done in 1951 by Carter et al., while Hood in 1971 published a review of the condition which was deemed to be the largest and most comprehensively done [12].
In Ghana, comprehensive data on diaphragmatic rupture are scanty. The only data available are two publications by Okyere et al., with one on right diaphragmatic rupture associated with hepatothorax and the other on left diaphragmatic rupture with herniated intrathoracic gastric perforation [13, 14]. Therefore, this study was aimed at evaluating the surgical management of traumatic diaphragmatic rupture in our institution over a ten-year period looking at the surgical approach and repair technique per the specialty that managed the case and the general outcome of the patients.
Aim
The aim of the study was to evaluate the surgical management experience of traumatic diaphragmatic rupture in our institution over a 10-year period in the local setting of a tertiary hospital in Ghana.
Material and methods
Study site/design
A cross sectional retrospective cohort study of the medical records of 35 consecutive patients who had undergone surgery for traumatic diaphragmatic rupture at the Komfo Anokye Teaching Hospital (KATH) between January 2010 and June 2020 was performed. KATH is the second largest hospital in Ghana, with a 1000-bed capacity and a recently established Cardiovascular and Thoracic Surgery Unit.
Collection of retrospective data
We manually collected the data of the 35 consecutive patients who had undergone surgery for diaphragmatic rupture between January 2010 and June 2020 at the Department of Surgery of the Komfo Anokye Teaching Hospital, Kumasi, Ghana. Patients’ data were retrieved from the operating theatre books and the patient’s folder, noting the demographics of the patients, the mechanism of injury, type of injury, cause of diaphragmatic rupture, type of surgical approach, type of anaesthesia, intraoperative findings and outcomes, and were analysed with Stata 13.
Data analysis
Data were analysed with Stata 13. Sociodemographic and clinical variables such as the mechanism of injury, type of injury, cause of diaphragmatic rupture, type of surgical approach, type of anaesthesia, intraoperative findings and outcomes were presented as means, ranges, frequency and percentages with simple tabulation.
Follow-up
Patients were followed up on an outpatient basis after discharge in 1 and 2 weeks and 1 month if there were no issues. The subsequent reviews were based on the clinical state indication.
Ethical approval
The Committee of Health Research Publications and Ethics of the Komfo Anokye Teaching Hospital approved the study with registration number: KATH IRB/AP/098/20. Data used were obtained in adherence to the principles of the World Medical Association Declaration of Helsinki and local regulatory requirements.
Results
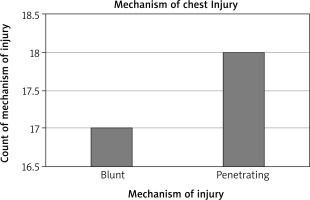
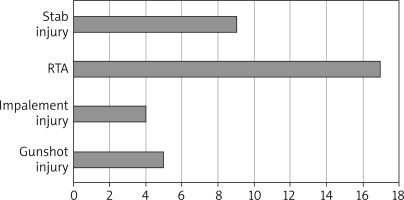
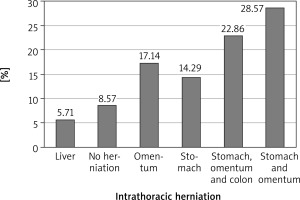
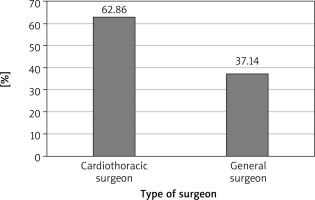
A total of 35 cases of diaphragmatic rupture were seen as a result of thoracoabdominal injuries. There were 29 (82.86%) males and 6 (17.14%) females. The mean age was 36.25 ±12.98 years with a range of 16–65 years. There were 3 cases of right diaphragmatic rupture and 32 cases of left diaphragmatic rupture. Penetrating chest injury caused 18 (51%) of the ruptures. The leading cause of injury was road traffic accident, which constituted 48.57%, closely followed by stab (25.71%), gunshot injuries (14.29%) and impalement injury (11.48%). Seventeen (49%) patients had their diaphragmatic ruptures repaired via laparotomy by general surgeons, mostly in the first 5 years in the absence of a cardiothoracic surgeon, and the remaining 18 (51%) were repaired mostly by the cardiothoracic surgeon via thoracotomy for the second 5 years. For the repair through thoracotomy, only 3 out of the 18 patients had double-lumen anaesthesia for single lung intubation, while the rest had conventional endotracheal intubation anaesthesia. The commonest herniated organ was the stomach, involving 24 cases, and the stomach was the only herniated organ in 5 of the cases. One patient died in the theatre from cardiac arrest after failed intubation.
Surgical technique
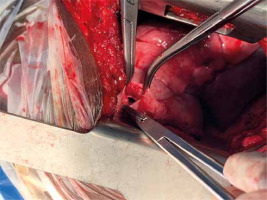
After informed consent, the cases that were surgically managed by the general surgeons were approached via a midline laparotomy under general anaesthesia. The diaphragmatic ruptures were repaired in a simple interrupted fashion using nylon 1. The abdomen, afterwards, was irrigated with warn normal saline and then closed up in layers. All the patients were extubated on table except 1 patient who had cardiac arrest after anaesthesia before surgery. Those who were operated by the cardiothoracic surgeon were approached via a standard posterolateral thoracotomy entering the 7th intercostal space or pleural bed after either conventional or double lumen endotracheal anaesthesia. The patients were positioned in a lateral decubitus position depending on the side of the rupture. Associated pleural collections such as haemothorax were sucked out after entering the chest cavity, the herniated intrathoracic organs were reduced into the abdomen and the edges of the ruptured diaphragm isolated and repaired in an interrupted, single layered fashion using nylon 1. Two patients who had intrathoracic herniated gastric perforation had repair of the stomach in the chest before being reduced into the abdomen. Any associated intrathoracic organ injuries such as lung laceration were also repaired. The thoracic cavity was then irrigated copiously with warm normal saline and closed up in layers after leaving a large chest tube, usually size 28FG or 32 FG. All the patients were extubated on Table I (Figures 1–9).
Table I
Patients’ demographic characteristics
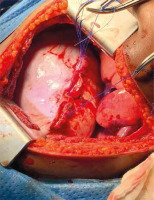
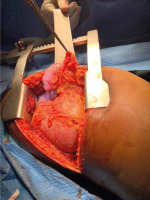
Figure 6
Intraoperative photograph showing a large diaphragmatic rupture with herniated spleen, liver and the greater omentum as seen through left thoracotomy
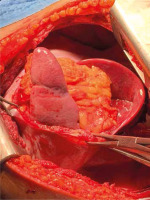
Figure 7
Another intraoperative photograph showing a large diaphragmatic rupture with herniated transverse colon, greater omentum and the stomach as seen via left thoracotomy
Discussion
Aetiology and classification
We observed a major male predilection in our study with a mean age of 36.25 years, which is consistent with other reported series such as that from Shah et al. These are people in their most productive ages and this has negative impact on the economy.
Blunt chest injuries have been found to be a more common cause of TDR than penetrating chest trauma with the main aetiologies being motor vehicle accident and falls from heights while penetrating injuries are commonly due to gunshot or stab wounds [1, 4, 5, 9, 11]. A study by Gelman et al. demonstrated that TDRs due to penetrating chest injuries usually measure about 2 cm and are mostly diagnosed clinically relying on the site of entry and trajectory of the wound. Blunt force injury-associated diaphragmatic ruptures, on the other hand, have tears measuring more than 2 cm, with most of them being above 10 cm and their diagnosis requiring high index of suspicion [15]. Diaphragmatic rupture from an iatrogenic causes, pregnancy-related and spontaneous diaphragmatic ruptures have also been documented [3]. Contrary to the above, we noted higher incidence of penetrating chest injury-associated diaphragmatic ruptures. Penetrating chest injury was responsible for the mechanism of injury causing the diaphragmatic rupture in 51% of the patients in our study. The leading type of injury was road traffic accident followed closely by stab and gunshot injuries. The impalement injuries causing diaphragmatic ruptures were all from knife injuries during fights or attacks.
There are several ways of classifying diaphragmatic ruptures. Firstly, they can be classified based on the mechanism of injury, where they are grouped into blunt and penetrating diaphragmatic ruptures. Secondly, they can be classified based on the laterality of the injury, into unilateral (left-sided or right-sided) or bilateral ruptures [12]. Thirdly, Shah et al. also reported a classification system for TDRs based on the clinical sequelae, into acute, latent and obstructive phases. The acute phase comprises the period following the initial trauma with accompanying multiple injuries. In the latent phase, there is abdominal visceral herniation through the undetected diaphragmatic defect while the obstructive phase is heralded by features of abdominal visceral (intestinal) strangulation [3, 16]. Fourthly, and based on the clinical sequelae of the diaphragmatic ruptures, they may also be grouped into acute or chronic diaphragmatic ruptures [12].
Left-sided diaphragmatic ruptures have been reported in several papers to be more common than right-sided ruptures, with reported incidences above 70% [5–8, 11]. This trend was similarly observed in our series, where only 3 (8.57%) out of the 35 patients had right diaphragmatic rupture, with left diaphragmatic rupture constituting 32 (91.43%). Of the three right-sided diaphragmatic ruptures, one was apparently from a blunt chest trauma to a woman who was accidently knocked down by a moving vehicle whiles crossing the road. She sustained serial rib fractures with right haemothorax and complete transverse rupture of the right diaphragm with herniation of the dome of the liver. The two other right diaphragmatic ruptures resulted from penetrating chest trauma. One was a knife stab wound to the right chest during a fight and the other was a male farmer who was accidentally attacked by the horn of a bull while returning from his farm. The bull lifted him up and stomped over him while on the ground several times on the right flank, resulting in laceration of the right diaphragm with intrathoracic herniation of the right lobe of the liver. The low prevalence of reported right diaphragmatic rupture has been postulated to result from the high mortality associated with it, giving rise to underdiagnosis [8]. Furthermore, the right hemidiaphragm is usually protected from the shock-absorbing liver. Moreover, an increased embryonic right hemi-diaphragmatic strength has also been reported. There are also reports of intra-pericardial and bilateral diaphragmatic ruptures with or without abdominal viscera herniation [6]. A review by Shah et al. reported 68.5% out of 980 cases being left-sided while 24.2%, 1.5% and 0.9% of cases consisted of right-sided, bilateral and intrapericardial diaphragmatic ruptures [3], respectively.
According to Taha et al., traumatic diaphragmatic injuries have been graded into five grades: Grade I – contusion of the diaphragm; Grade II – diaphragmatic laceration up to 2 cm; Grade III – laceration of 2–10 cm; Grade IV – laceration > 10 cm or injury with tissue loss up to 25 cm3; Grade V – tissue loss greater than 25 cm3.
Mechanism of injury
Just as was observed in our series, stab and gunshot injuries are reported to be the most common causes of penetrating diaphragmatic rupture, especially when the injury is at the level of the fourth intercostal space and below due to the ascent of the diaphragm on full expiration [2]. However, in blunt diaphragmatic injuries, the sudden impact to the abdomen causes an increase in intraperitoneal pressure which is transmitted through the hemi-diaphragms and causes abrupt rupture and possible herniation of the abdominal viscera through a congenital weakness or diaphragmatic injury from the ends of the fractured ribs [2, 3].
Clinical presentation and mortality
Clinically, diagnosis of TDR is difficult since there are no specific clinical symptoms and signs for diaphragmatic rupture [11]. However, there may be complaints of epigastric or hypochondriac pain with dyspnoea. Features associated with the acute phase of diaphragmatic rupture as reported by Shah et al. may include non-specific abdominal pain, especially in the left upper quadrant or shoulder tip pain. It may be accompanied by dyspnoea, orthopnoea or cyanosis and hypotension in the event of massive herniation with mediastinal shift [12]. In the latent phase, otherwise known as the interval phase, symptoms such as left upper quadrant pain or features of intermittent intestinal obstruction, especially following meals, which tend to resolve with belching, passing flatus or vomiting, may be experienced in some patients [3, 12]. The obstructive phase symptoms include features of intestinal herniation and obstruction with possible strangulation. It may include vomiting (projectile in gastric outlet obstruction), nausea and severe abdominal pain. In the event of a possible strangulation and intra-thoracic bowel perforation, there may be complaints of pleuritic chest pain with feco-pneumothorax and pleurisy [10]. Similar to Ganie et al., who reported 2 cases of diaphragmatic injury with colonic herniation and perforation into the thoracic cavity [17], 2 of our patients sustained left diaphragmatic rupture with intrathoracic gastric perforation and the repairs of the perforation were done in the chest before reducing the stomach back into the abdomen. One of these patients had extensive mediastinitis with frozen thoracic cavity because of delayed diagnosis after initially missing the rupture during an earlier exploratory laparotomy, but the patient survived. The most commonly herniated intrathoracic intestinal organ was the stomach, involving 24 cases, with the stomach being the only herniated organ in 3 of the cases in our series, as shown in Figures 4 and 5. Five patients had no intrathoracic viscus/organ herniation. Auscultation of bowel sounds in the lower lung zones with dull percussion note may also be suggestive of a possible diaphragmatic rupture with bowel herniation [7, 10, 13]. The preoperative diagnosis of most of our patients was of clinical suspicion, with the most common imaging done being chest X-ray. Few patients who were initially stable and had concomitant abdominal signs had CT scan of the chest and the abdomen done. The woman with a right diaphragmatic rupture from vehicular knockdown had confirmation of the diagnosis by CT scan.
Shah et al. reported that diaphragmatic rupture is almost certainly always associated with other injuries that tend to delay the diagnosis. Commonly implicated associated injuries include shock, respiratory distress and coma [3]. It has been reported that up to two-thirds of patients with TDR and associated multisystem injuries have had initial diagnosis of the rupture missed, and this accounts for the drastic rise in mortality and morbidity rates [13]. At least 62% of patients with associated injuries have been documented to have had diagnosis of diaphragmatic rupture missed up until the 4th day [3]. Asymptomatic cases especially may be seen in patients with small tears with no intrathoracic herniation [7], as shown in Figure 3.
Most cases of TDR are commonly missed, with early and accurate diagnosis made in less than 50% of cases [7]. Shah et al. also quoted similar figure, where only 43.5% of their cases were accurately diagnosed preoperatively. About a further 40% of them were diagnosed either at autopsy or intraoperatively, while 14.6% of cases had delayed diagnosis [3]. De Lesquen et al. reported that about a fifth of their cases are diagnosed after 48 hours [18]. This difficulty in diagnosis accounts for the associated high morbidity and mortality. A mortality rate of 30% has been quoted in patients with strangulation of the herniated bowel [7].
Diagnostic investigation
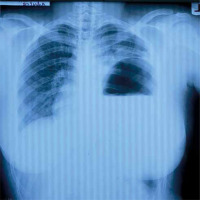
Chest radiography is the initial imaging study employed in the diagnosis of diaphragmatic rupture. Sensitivities of 27–60% for left-sided ruptures and 17% for right-sided ruptures have been documented [7]. Al-Refaie et al. also reported similar sensitivities of 30–62% in the event of absent abdominal visceral herniation and over 90% in cases associated with herniation [11]. De Lesquen et al. reported that 30% of cases of traumatic diaphragmatic rupture are missed on chest X-ray. However, Adegboye et al. in a review of treated diaphragmatic injuries stated that chest X-ray diagnosed diaphragmatic rupture in about 70% of cases in which it was due to blunt chest injury but was useful in only half of patients with diaphragmatic rupture due to penetrating chest injury [4]. Radiological features of diaphragmatic rupture on chest X-ray include presence of gastric bubble in the hemithorax as shown in Figure 6. Other signs may include the presence of a radio-opaque nasogastric tube in an abdominal viscus in the hemithorax, obliteration of the diaphragmatic outline or distortion of its contour, elevation of the hemidiaphragm, pleural effusion or air-fluid level in the lower thorax, and contralateral shift of the mediastinum [2, 3].
CT scan is the gold standard in diagnosing diaphragmatic rupture [16]. Sensitivity and specificity of 61–71% and 87–100% respectively have been quoted in its use in diagnosing diaphragmatic rupture [5]. Sensitivity is even higher when a helical CT is used [11]. Features such as ‘collar sign’, ‘dependent viscera sign’ and ‘hump sign’ are features of diaphragmatic rupture. In the event of an equivocal finding on CT, magnetic resonance imaging (MRI) showing sagittal views can be employed [6]. As seen in a case report by Lone et al., about 50% of all cases of diaphragmatic rupture are first diagnosed intra-operatively during surgery for other associated injuries [4, 5].
Other radiological techniques that can be employed in diagnosis include ultrasonography, barium swallow or contrast studies and scintigraphy [19]. Ultrasonography may demonstrate diaphragmatic tears or abnormal diaphragmatic movements. It is particularly beneficial since it can easily be carried out in the emergency room as part of resuscitation. However, its usefulness in accurately diagnosing diaphragmatic rupture has been conflicting among the few studies carried out on it. The discrepancy has been suspected to be due to operator-dependent differences [2].
In penetrating diaphragmatic injuries, the use of digital exploration as a diagnostic method was thoroughly studied in a cohort study by Morales et al. In that study, patients with penetrating chest injuries suspected to have associated diaphragmatic injury were selected and digital exploration of the wound tract with the index finger after administration of local anaesthesia was carried out. It was found that 51 out of 82 patients (62.2%) who underwent digital exploration had diaphragmatic injuries, of which 50 were indeed confirmed to have a perforated diaphragm at laparotomy. All 25 patients who were negative on digital exploration were confirmed at thoracoscopy. The study was found to have a 96% sensitivity, 83.3% specificity, a 91% positive predictive value and a documented 93.7% negative predictive value [20].
In recent times with the advent of minimally invasive techniques such as thoracoscopy and laparoscopy has led to their use in diagnosing and in some cases treating some diaphragmatic injuries. No particular differences have been found in their accuracies. However, they are particularly not useful in acute trauma, especially in a patient who would require laparotomy [2].
Management
Diaphragmatic ruptures, as a general rule, require surgical repair irrespective of the length of the tear and the time of diagnosis. Documented reasons for requiring surgical repair include the difficulty in the healing of the tear due to the constant diaphragmatic motion. Also, any increase in abdominal pressure such as coughing or straining could cause worsening of the tear or herniation of abdominal viscera into the thorax. The choice of surgical approach has remained controversial, with no current evidence supporting one over the other [16], and this forms one of the bases of this paper.
Surgical approaches for the repair of diaphragmatic rupture include laparotomy, thoracotomy, combined laparotomy and thoracotomy, thoracoabdominal, laparoscopy and video-assisted thoracic surgery (VATS) or thoracoscopy. The choice of approach depends on several factors including the type of injury, such as acute or chronic, the laterality of the injury (right or left), the presence or absence of associated abdominal or thoracic injury, expertise (general surgeon, trauma surgeon or cardiothoracic surgeon), availability of equipment and the cavity with the severe associated injuries or significant haemorrhage. Intra-abdominal bleeding giving rise to massive chest tube output or urohaemothorax in the case of ruptured urinary bladder may lead to the wrong cavity being opened first. The cavity to be opened first, thorax or abdomen, is often a challenge in such cases. Therefore, at times thoracoabdominal injuries may require surgical intervention in both the chest and the abdomen. In considering the surgical approach to the repair of a ruptured diaphragm, the presence or absence of concomitant injures must be well established, as noted earlier, since isolated diaphragmatic ruptures occur in less than 10% of cases [2]. Isolated diaphragmatic injuries can be managed via minimally invasive techniques such as thoracoscopy or laparoscopy, and such approaches are particularly useful in diagnosing and repairing small tears [2]. The options for larger defects with significant visceral herniation are primarily between laparotomy and thoracotomy, as employed by several studies [2, 4, 6, 8, 16]. Several case series have reported different preferences. Studies by Gao et al. and Turhan et al. showed that most cases of diaphragmatic rupture were repaired by laparotomy. Gao et al. postulated that laparotomy was the optimal approach in diaphragmatic rupture due to blunt chest injury as a result of the frequent co-existence of abdominal injuries, and this has been echoed in other literature [2, 6]. Thoracotomy was the preferred option for penetrating chest injuries so as to adequately control any associated intra-thoracic vessel injury or lung lacerations [6]. Turhan et al. reported that laparotomy was preferred for acute cases in order to address any associated intra-abdominal injuries while thoracotomy was optimum for late cases so as to adequately free any adhesions that may have formed. Comparably to our series, where 51% of the cases were repaired via thoracotomy, thoracotomy was found to be the most preferred option for surgery in the study by Adegboye et al., with about 60% of the cases repaired being done via thoracotomy. It has also been documented to be absolutely indicated in the management of right-sided ruptures irrespective of the duration of injury due to the ease of repair [12], as was employed in our 3 cases of right diaphragmatic rupture.
Another school of thought on the choice of approach for repair was reported by Ganie and co-workers, who found that the specialty department in charge of managing the case determined the approach employed, a postulation entertained by us also in our series. In the first 5 years of this study, which coincides with the period before the arrival of the cardiothoracic surgeon, the general surgeons handled most of the cases. Only two of those cases within that period were managed by the cardiothoracic surgeon while on vacation. However, the cardiothoracic surgeon had handled almost all the cases in the last 5 years of the study period. It was reported that 92% of cases managed by general surgeons were approached via laparotomy whereas the thoracic surgeons preferred thoracotomy in 78% of their cases managed [15], and therefore the cardiothoracic surgeon usually prefers to repair the diaphragm through the chest via thoracotomy, whereas the general surgeons prefer laparotomy. However, there are few absolute indications for a thoracotomy approach in diaphragmatic ruptures, and these include injury involving the thoracic aorta, thoracoabdominal impalement injury, pericardiodiaphragmatic rupture, a severe contamination of the thoracic cavity, a right diaphragmatic rupture as noted above and, finally, a chronic or delayed diagnosis.
In spite of the myriad views on the choice of approach for repair of diaphragmatic injuries, the mode of closure of the tear has remained unanimously accepted. Continuous or interrupted suturing of the diaphragmatic tear with a non-absorbable suture is recommended by all [2, 8]. In cases of large diaphragmatic defects which cannot be closed primarily, a graft from the fascia lata, pericardium (human, porcine or bovine) or synthetic material such as Gore-Tex, Dacron or commonly mesh may be used to close the defect [12]. Though we had a number of large defects, all of them were repaired primarily without the use of any native or synthetic material. All the repairs were also done in simple interrupted fashion using nylon 1, by either the cardiothoracic or the general surgeon, as shown in Figure 9.
Limitation from the small sample size of our series. Data mainly from the theatre records of the patients
Conclusions
The diagnosis of diaphragmatic rupture can be a common occurrence in thoracoabdominal injuries, whether blunt or penetrating. Penetrating injury is the leading cause of rupture. It commonly involves the left hemidiaphragm. Surgery is the treatment of choice and it is repaired via laparotomy or thoracotomy based on the presence or absence of concomitant abdominal injury, the side of the rupture and the presence or absence of a cardiothoracic surgeon.