Introduction
Primary cardiac tumors are a group of rare diseases with incidence of 0.001 to 0.3% [1]. Seventy-five percent of them are benign, mostly represented by myxoma [2]. They present with a wide variety of symptoms (breathlessness, arrhythmias, congestive heart failure, central and peripheral embolization, etc.). However, most of the time they are found accidentally while examining for another disease [3]. The leading treatment modality was surgical resection. Long-term prognosis of patients was reported to be good if the tumor was excised completely [4].
Malignant tumors comprise 15% of all primary cardiac tumors [5]. The most frequent type is sarcoma, usually diagnosed at around 40 years of age [6]. The symptoms include tachyarrhythmias, heart conduction disorders and pericardial effusion. They are caused by direct invasion into the myocardium of all heart chambers. If the tumor affects the function of heart valves, it can present with congestive heart failure [7]. The prognosis is poor as long affects as most of the patients already have distant metastases at the time of diagnosis. The postoperative survival is 3 to 12 months despite the combination of surgical and adjuvant oncological treatment [8].
Aim
Although the outcomes of cardiac oncosurgery were reported to be favorable, there is only scarce evidence coming from the recent era [1, 4, 7–12] and from the central Europe [13]. Therefore, we aimed to analyze the short- and the long-term outcomes of patients undergoing surgery for primary cardiac tumor at our department throughout the last two decades. We also compared the survival of study cohort with that of the general population.
Material and methods
We performed a retrospective analysis of perioperative and long-term outcomes of patients undergoing surgery for primary cardiac tumor at our department between 2000 and 2020. The perioperative data were manually extracted from the in-hospital patient records. The data on long-term survival and reoperations were provided to us by the National Registry of Cardiac Surgery administered by the Institute of Health Information and Statistics of the Czech Republic. It is compulsory for all cardiac surgery centers in the country to regularly provide the data on all cardiac procedures to this registry. The registry of deceased persons is directly connected to it. While the observed long-term outcomes were taken only from the compulsory national registry, we considered the long-term follow-up 100% complete. Individual informed patient consent was waived. The study was approved by the institutional ethics committee (9th of September 2021, Reference number 202109 P04). The data underlying this article will be shared on reasonable request to the corresponding author.
Surgical technique
The surgery was performed through the standard median sternotomy approach in most of the cases. In 2 patients, the right anterolateral minithoracotomy was chosen. All operations were performed on mild hypothermic cardiopulmonary bypass with cardioplegic cardiac arrest. The approach to the left atrium was through the interatrial groove in all cases where necessary. Maximum care was taken to excise the tumor completely with its stalk and adjacent tissue, to provide radical resection. Reconstruction of the interatrial septum with a pericardial patch was performed where necessary.
Statistical analysis
The perioperative outcomes were analyzed using the standard descriptive statistic methods in Microsoft Excel 365 (Washington, United States of America, 2021). The categorical data are presented as numbers and percentages. The continuous data were tested for normality using the Shapiro-Wilk test. Most of them did not have normal data distribution; therefore the data are presented as medians and interquartile ranges. The study cohort was afterwards compared with a general age- and sex-matched population using relative survival analysis with R (The R Foundation for Statistical Computing, Vienna, Austria, version 4.0.3) in RStudio (RStudio, Inc., Version 1.2.5042) using the Pohar Perme method, relsurv R package (Perme, 2018) version 2.2-3. The resulting values express the relative survival difference of the study group against general population at pre-specified timepoints together with 95% confidence intervals (CI). The survival difference was considered statistically significant when the CI did not contain the 100% value. The Kaplan-Meier survival analysis was performed using the R packages survival version 3.2-7 (Therneau, 2020) and survminer version 0.4.8 (Kassambara et al., 2020).
Results
Throughout the study period, 60 patients underwent surgical resection of a primary cardiac tumor at our institution. Four types of tumors were observed: myxoma (n = 53; 88%), papillary fibroelastoma (n = 5; 8%), epicardial lipoma (n = 1; 2%) and undifferentiated sarcoma (n = 1; 2%). The complex description of observed cardiac tumors together with rich histopathological illustration is provided in Online Supplementary Material [14–17].
The myxoma was mostly located in the left atrium (n = 49; 92%) followed by the right atrium (n = 4; 8%). Papillary fibroelastoma was seen on the aortic valve (n = 4; 80%) or at the interventricular septum from the left side (n = 1; 20%). Epicardial lipoma was extremely rare and was diagnosed accidentally during the coronary artery bypass grafting. One patient underwent the surgery for malignant primary cardiac tumor, and thus the undifferentiated sarcoma of the left atrium.
Clinical symptoms associated with the tumors were breathlessness (n = 31; 52%), systemic embolization into the central nervous system, retina, spleen or lower limb (n = 10; 17%), palpitations (n = 5; 8%), syncope (n = 5; 8%), malnutrition (n = 2; 3%) and other rare symptoms in single patients (pulmonary embolism, abdominal pain, night sweats). Thirty-three percent of patients also underwent concomitant cardiac surgery for coronary artery disease or heart valve disease. See Table I for detailed preoperative cohort characteristics.
Table I
Preoperative patient characteristics
We observed early postoperative mortality in 2 patients. Both of them were fragile, over 80 years of age. The first patient, after left atrial myxoma resection, died on postoperative day 14 because of uncontrollable bleeding from the right ventricle and brachiocephalic vein after surgical evacuation of pericardial effusion. The second one had combined cardiac surgery (left atrial myxoma resection, aortic valve replacement, septal myectomy) and died on postoperative day 2 because of cardiogenic shock. Post-mortem diagnostics showed a thrombosis of the circumflex artery and pulmonary embolism as underlying causes.
The dominant postoperative complication was new onset of atrial fibrillation; only 2 patients of those also had a preoperative history of tachyarrhythmia. The second most common complication was temporary conduction block; 2 patients required implantation of a permanent pacemaker due to junctional bradycardia. Other complications were relatively rare (Table II).
Table II
Perioperative outcomes
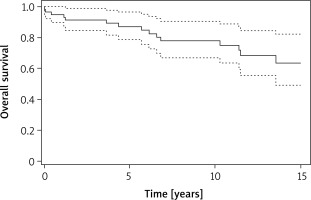
Mean postoperative follow-up was 8.5 years. We recorded 13 long-term deaths throughout the study period (Figure 1). One patient died as a consequence of metastatic malignant primary cardiac tumor 15 months after the surgery, and the remaining patients died for reasons not associated with heart tumor. According to Kaplan-Meier analysis, the estimated long-term survival was 0.87 (95% CI: 0.79–0.97) at 5 years, 0.78 (95% CI: 0.67–0.91) at 10 years and 0.64 (95% CI: 0.49–0.82) at 15 years.
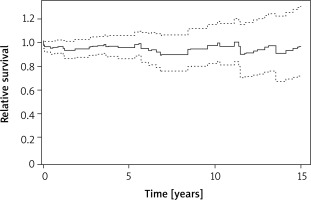
The long-term postoperative survival was equal to the age- and sex-matched general population (Figure 2). The relative survival was 0.96 (95% CI: 0.87–1.06) at 5 years, 0.90 (0.76-1.06) at 10 years and 0.91 (95% CI: 0.68-1.23) at 15 years.
Figure 2
Long-term relative survival comparison of the study cohort with age- and sex-matched general population
Recurrence of the tumor was only seen in 4 (2%) patient with myxoma at the lateral wall of the left atrium. He underwent an uneventful reoperation 6 years later; the recurrent myxoma was found in the area of the pulmonary veins.
Discussion
Around 800 cardiac operations are carried out every year at our department comprising the whole spectrum of adult cardiac procedures. The cardiac tumors represent a minor part of them, up to three procedures per year. The most common type of benign primary cardiac tumor is myxoma, as seen in our cohort as well as the reports of others [11, 13]. The clinical symptoms depend on the size, character and location of the tumor. A typical clinical presentation is breathlessness caused by obstruction of the mitral valve orifice. The patients are at risk of acute heart failure and sudden cardiac death. The myxoma also has a high emboligenic potential [18]. Embolizations are particularly frequent in myxomas of the mitral valve, more than in left atrial wall myxomas, due to valve movement during the heart cycle [19]. Papillary character of the tumor may also lead to higher risk of embolism [9, 20]. In concordance with others, 70% of embolizations in our cohort were seen in papillary tumors.
The second most common benign cardiac tumor is papillary fibroelastoma [10]. It is characterized by slow growth and is located mostly on the aortic valve, followed by the mitral valve, interventricular septum or tricuspid valve. Most of the patients are asymptomatic. Nevertheless, the tumor has a high emboligenic potential due to the fragile character of its tissue and the risk of thrombus formation. Usually they embolize to the central nervous system or coronary arteries [21]. For this reason, a surgical resection is indicated. In small asymptomatic fibroelastomas, however, regular echocardiographic follow-up and anticoagulation treatment may be considered [22]. In our cohort we did not record a history of embolization in patients with fibroelastoma. The patients presented mostly with heart failure of New York Heart Association class II-III.
Cardiac lipoma is a rare tumor with the incidence below 0.1% in the adult population [23]. It is mostly located in the epicardium but may occur in any heart chamber. Usually, it is found accidentally, as seen also in our cohort. The clinical symptoms comprise arrhythmias, heart failure (caused by pression on any heart chamber), or sudden cardiac death. When diagnosed, surgical resection is indicated.
As reported by previous evidence, malignant tumors represent one fourth of primary cardiac tumors [17]. The most common type is sarcoma, mostly angiosarcoma. The prognosis of patients with malignant heart tumor is generally poor. It depends on the excess of disease, presence of metastases, and on the possibility of surgical resection. Late diagnosis is common as long as the clinical symptoms are not specific or are not present at all until the terminal stage. Estimated survival is up to 12 months [24]. Adjuvant chemotherapy is the treatment of choice in the context of palliative care. The best outcomes are seen in patients after complete tumor resection, adjuvant chemotherapy and radiotherapy [25]. In contrast with the literature, the incidence of primary cardiac malignancy was extremely low in our experience. We saw only a single case of an undifferentiated sarcoma whose long-term survival was poor. Although primary cardiac malignancies are rare, metastatic heart disease is a frequent condition seen in up to 20% of patients dying of malignancy [26].
There is a well-known trend towards reduction of invasiveness in cardiac surgery. This has also extended into cardiac oncosurgery, mostly utilizing the right anterolateral minithoracotomy approach [12]. Detailed tumor imaging, evaluation of its anatomy and proximity to surrounding structures with sonography, computed tomography or magnetic resonance is crucial in preoperative planning and choosing the correct surgical strategy. In our experience we performed two procedures of minimally invasive tumor resection through minithoracotomy with acceptable outcomes.
As documented in the reports of others, the long-term survival of patients with a benign primary cardiac tumor is favorable [1, 4, 13]. It is not affected by the diagnosis of tumor and is comparable with the age- and sex-matched general population, particularly in myxomas [4]. Our study confirms these results and supports the surgical strategy as the treatment of choice. As stated above, the prognosis of malignant cardiac tumors remains poor despite optimal treatment.
Our study has some important limitations. Firstly, it was a retrospective observational single center study and the number of patients was relatively low. The long-term data were provided by the national registry. We recorded only a single case of sarcoma; therefore we were unable to better elaborate on this.
On the other hand, the strengths of the study are as follows: (i) it is one of the largest recent single-center studies on primary cardiac tumors published having a relatively long follow-up; (ii) it supports the fact that the surgical resection is the treatment of choice with excellent long-term outcomes in the contemporary era; (iii) it provides a detailed analysis of preoperative symptomatology and perioperative outcomes in cardiac oncosurgery together with rich histopathological descriptions and illustrations.
Conclusions
Primary cardiac tumors are relatively rare. Although mostly benign, they may present with a wide variety of clinical symptoms and severe morbidity. Surgical resection is the treatment of choice. It is associated with favorable short- and long-term results. When anatomically feasible, it may be performed in a minimally invasive fashion with acceptable outcomes. The recurrence of benign heart tumors after successful resection is rare and resection the long-term mortality is not influenced by the diagnosis of cardiac tumor. Malignant cardiac tumors are a lethal condition with poor prognosis and reduced survival despite maximum treatment.