Introduction
Chronic hepatitis B (CHB) is still an important public health problem; worldwide 330 million people are infected with this virus [1, 2]. Its clinical course is not homogenous in all patients; some individuals carry this virus throughout their life, but others develop cirrhosis and hepatocellular carcinoma (HCC). Current treatment options can only stop disease progression, rather than viral eradication. As noted above, HCC is an important consequence of hepatitis B virus (HBV); it develops in HBV-related CHB and also in HBV-related cirrhosis [3]. Also, HCC can be detected in inactive HBV carriers, the incidence of HCC in this group of patients is less than 0.3% per year. It is important to recognise HCC in its early stage in terms of improved survival with effective HCC treatments [4].
Survivin is an anti-apoptotic protein, which was first discovered in 1997 [5]. Subsequently, studies disclosed its structure and function. Survivin, a 142 amino acid containing protein, is a member of the inhibitor apoptosis protein (IAP) family. Its gene is located at 17q25 and is expressed in the G2/M phase [6]. Some protein members of IAP family expressed in both embriyonic and terminally differentiared tissues [7, 8]. It was demonstrated by using northern hybridisation that adult liver, spleen, pancreas, kidney, and other visceral organs did not express survivin [9]. Survivin expression is undetectable in all terminally differentiated tissues except thymus, testis, vascular endothelial cells, and CD34+ stem cells. Nowadays, it is well known that this protein plays a major role in many malignancies, including gastric cancer, lung cancer, colon cancer, and ovarian cancer [10]. Survivin expression is associated with advanced stage and poor differentiation in malignancies [11].
In the literature, most of the studies investigate survivin in HCC as a potential stimulator of malignancy. Nevertheless few studies investigate survivin expression in CHB. In 2000, Ito et al. demonstrated that survivin promotes tumoural cell proliferation in HCC [12]. After that many studies revealed survivin expression in HCC. In the view of this information, we hypothesised that survivin expression can start in the liver of CHB patients before the cirrhotic situation and HCC.
Material and methods
Study design and tissue sampling
This is a single-centre retrospective cross-sectional study. Seventy-five CHB patients and eight control patients were enrolled into the study between 2008 and 2018. Liver biopsy was performed in all CHB patients for documentation of liver necro-inflammatory activity and fibrosis before initial medical treatment. The control group comprised cholecystectomy materials with intact liver tissue without any proof of acute and/or chronic inflammatory liver disease. Eight laparoscopic cholecystectomy patients fulfilled these criteria.
Liver biopsies were processed in an automated tissue processor and placed in tissue paraffin blocks. Haematoxylin and eosin (H&E)-stained slides were prepared for biopsy samples. The biopsy specimen was considered sufficient if it comprised 6–8 portal spaces. All liver biopsy specimens were evaluated by the same pathologist, who was experienced in liver pathology and blinded to the clinical data of the patients. In CHB patients, liver pathology was performed with the Ishak scoring system. According to modified Hepatitis Activity Index (HAI), patients were subdivided into three groups based on their scores: minimal HAI with 1–3 points, mild HAI with 4–6 points, and moderate HAI with 9–12 points. Bridging fibrosis describes a fibrosis score of 3 or 4.
Immunohistochemical staining and immunostaining evaluation
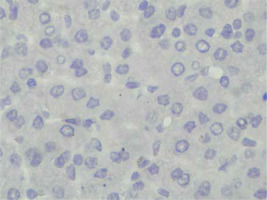
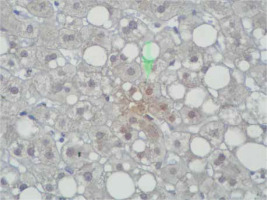
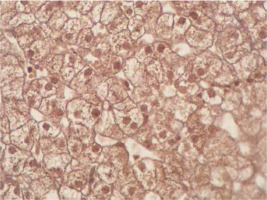
Immunohistochemical staining of sections from the liver tissue samples was performed using a Ventana BenchMark GX (Roche Diagnostics, Basel, Switzerland). In brief, after deparaffinisation using EZ-Prep (Roche Diagnostics) and antigen retrieval using Cell Conditioning 1 buffer at 95°C for 32 min, sections were incubated with primary antibodies. The primary antibodies were anti-survivin (cat. no. ABP54792, polyclonal, dilution 1 : 200; R&D systems, California, USA). Immunoreactivity was considered based on nuclear staining and by counting 100 hepatocytes under a high-power field. The severity of immunoreactivity was assigned by percentage as positive cell number: Grade 0: no staining (Figure 1), Grade 1: 1–20% (Figure 2) and Grade 2: > 20% staining (Figure 3).
Statistical analysis
The normality of distribution of continuous variables was tested by Shapiro-Wilk test. Mann-Whitney U and Kruskal-Wallis tests were used for comparison of non-normal numerical variables between two or three independent groups, respectively. Dunn’s multiple comparison test was applied when the Kruskal-Wallis test result was significant. The χ2 test was applied to investigate the relationship between two categorical variables, and Spearman rank correlation coefficients were used to assess the relation between non-normal numerical variables. Statistical analysis was performed with SPSS for Windows version 24.0, and a p-value < 0.05 was accepted as statistically significant.
Results
A total of 75 CHB patients were enrolled into the study. Mean age was 45.95 ±10.13 years, 36 patients were female, and 39 patients were male. Between the groups, there is no statistically different according to sex and age (p = 0.436 and p = 0.350, respectively). Most of the patients (n = 70) were HbeAg–/AntiHbe+, and the rest were HbeAg+. Mean levels of initial laboratory parameters were 60.30 ±80.88 for alanine aminotransferase (ALT), 45.02 ±65.73 for aspartate aminotransferase (AST), 2.03 ±1.44 for α-fetoprotein (AFP), and 2292.2 ±2146.45 for HbsAg. In standard pathological examination, according to HAI, most of our patients were mild. The mean HAI score was 4.87 ±2.04. Also, fibrosis was generally mild and, in some cases, moderate, with a mean score off 1.12 ±0.96. Demographic characteristics, baseline laboratory parameters, and initial pathological findings are listed in Tables I and II.
Table I
Demographic characteristics and initial pathology features of patients
Table II
Mean values of baseline laboratory parameters and initial pathology features of patients
Survivin immunoreactivity was observed in 46 patients in CBH; nevertheless, pathology preparations were not stained by anti-survivin antibody in 29 patients. In 46 patients, immunoreactivity was observed as grade 2 in 14 patients and grade 1 in 32 patients. On the other hand, only 1 patient’s preparation showed immunoreactivity in the control group. Between patients and control groups, the presence of survivin immunoreactivity and severity was significantly higher in CHB patients compared to controls (p = 0.008 and p = 0.028, respectively) (Table III).
Table III
Comparison of survivin immunoreactivity and severity of survivin immunoreactivity between chronic hepatitis B patients and controls
| Variable | Groups | P-value | ||||
|---|---|---|---|---|---|---|
| CHB | Control | |||||
| n | % | n | % | |||
| Survivin immunoreactivity | Presence | 46 | 61.3 | 1 | 12.5 | 0.008* |
| Severity of survivin immunoreactivity | Absence | 29 | 38.7 | 7 | 87.5 | |
| Below 20% | 32 | 42.7 | 1 | 12.5 | 0.028* | |
| Above 20% | 14 | 18.7 | 0 | 0.0 | ||
In CHB patients, we compared HAI, the presence of fibrosis, and bridging fibrosis between patients in whom survivin immunoreactivity was positive and those in whom it was negative. There was no significant difference of these parameters between two groups. In addition, we also evaluated HAI, the presence of fibrosis, and bridging fibrosis according to the severity of survivin immunoreactivity. HAI and the presence of fibrosis were not significantly difference between the grade 1 and grade 2 groups. However, bridging fibrosis was significantly higher in the grade-1 group compared to others (p = 0.027) (Tables IV, V). We also compared baseline laboratory parameters such as ALT, AST, AFP, HbsAg, and HBV-DNA between anti-survivin stain-positive and anti-survivin stain-negative groups. According to this comparison, no significant difference was found between these parameters (p = 0.011 for ALT, p = 0.957 for AST, p = 0.327 for AFP, p = 0.101 for HbsAg, and p = 0.948 for HBV-DNA).
Table IV
Comparison of HAI, presence of fibrosis, and bridging fibrosis according to survivin immunoreactivity
| Parameter | Survivin immunoreactivity | P-value | ||||
|---|---|---|---|---|---|---|
| Presence | Absence | |||||
| n | % | n | % | |||
| HAI | Minimal | 15 | 32.6 | 8 | 27.6 | 0.725 |
| Mild | 28 | 60.9 | 20 | 69.0 | ||
| Moderate | 3 | 6.5 | 1 | 3.4 | ||
| Fibrosis | Presence | 31 | 67.4 | 21 | 72.4 | 0.646 |
| Bridging fibrosis | Presence | 5 | 10.9 | 0 | 0.0 | 0.066 |
Table V
Comparison of HAI, presence of fibrosis, and bridging fibrosis according to survivin immunoreactivity severity
| Parameter | Severity of survivin immunoreactivity | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Absence | Below 20% | Above 20% | ||||||
| n | % | n | % | n | % | |||
| HAI | Minimal | 8 | 27.6 | 9 | 28.1 | 6 | 42.9 | 0.561 |
| Mild | 20 | 69.0 | 20 | 62.5 | 8 | 57.1 | ||
| Moderate | 1 | 3.4 | 3 | 9.4 | 0 | 0.0 | ||
| Fibrosis | Presence | 21 | 72.4 | 22 | 68.8 | 9 | 64.3 | 0.860 |
| Bridging fibrosis | Presence | 0 | 0.0 | 5 | 15.6 | 0 | 0.0 | 0.027* |
In this study we also evaluated the correlation between survivin immunoreactivity, baseline laboratory parameters and initial pathology parameters. When we investigate the correlation between the severity of surviving immunoreactivity and laboratory parameters such as AFP, HBV-DNA, HbsAg, ALT, and AST, we did not find a significant correlation between these parameters (r = –0.020 and p = 0.862 for ALT, r = –0.061 and p = 0.606 for AST, r = –0.046 and p = 0.697 for AFP, r = 0.147 and p = 0.207 for HbsAg, and r = –0.021 and p = 0.859 for HBV-DNA).
Discussion
Chronic hepatitis B is a well-known pre-malignant condition. In chronic hepatitis phase and cirrhotic phase, affected liver tends to develop HCC. In the literature many studies with variable results have investigated this tendency. These studies mainly focus on mutations and intracellular cascades, which can lead to malignancy. Survivin expression has not been investigated widely in CHB patients. In this study, we are the first according to the literature to show expression of survivin in the liver of CHB patients.
Chronic hepatitis B leads carcinogenesis in many ways, and chronic inflammation is one of them. Chronic inflammation concomitantly increases oxidative stress and radical oxygen species (ROS). It is well known that ROS causes DNA damage, which is a contributor of HCC development. HBV genome insertion to the host DNA makes hepatocytes more vulnerable to various mutations including tumour suppressor genes [13]. Overall, HbxAg is the key factor and plays a central role in the development of HCC. HBV genome encodes four overlapping open reading frames (ORF), of which X ORF is one [14]. X ORF encodes a 16.5 kDa protein, HbxAg, which has multiple functions. This protein contributes to chronic hepatitis and plays a role in the development of hepatocarcinogenesis in many possible ways [15]. One possible mechanism is activating transcription; Hbx protein elevates the levels of phosphorylated Raf and GTP-bound Ras, which eventually leads to HCC [16]. Hu et al. demonstrated that HBx protein increases cell proliferation via lncRNA UCA1 [17]. Survivin protein is another important target of HBx protein on the way to hepatocarcinogenesis. Zhang et al. revealed that HBx protein accelerates survivin expression in HCC [18]. So, it is possible to conclude that HBx protein and survivin have an interaction under the circumstances of malignancy, and this interaction may sprout in the chronic hepatitis phase, when we look at our results. But this speculation needs to be confirmed with studies that investigate HBx protein-survivin interaction in the CHB phase.
Survivin expression in HCC is well documented, and its role is almost completely understood. First of all, survivin inhibits apoptosis in HCC with many ways. Inhibiting caspase activity and binding to hepatitis B X-interacting protein leads its anti-apoptotic effect [18]. Beyond its anti-apoptotic effect, surviving can induce HCC cell proliferation [19]. Also, its expression gains radiotherapy and drug resistance to HCC. Overall its expression negatively affects HCC prognosis. Its crucial role in HCC development and progression makes it an important therapeutic target. Experts concluded that inhibiting surviving can inhibit HCC development and/or progression. Therefore, in vitro and animal studies were performed to investigate molecules that can inhibit the survivin effect [20].
In the course of HCC development, we do not really know when survivin expression starts; in dysplastic nodules, in mature HCC tissue, or in advanced fibrosis? Here we demonstrated that survivin was expressed in the liver of CHB patients. In contrast to our results, Ito et al. showed that 8 patients with cirrhosis and chronic hepatitis did not express survivin [12]. Nonetheless they did not give any information about their causes of these 8 patients. It is important to understand and demonstrate survivin expression in both chronic hepatitis and cirrhotic phases of CHB infection in terms of understanding HCC development and possible drug therapies.
Our study has some limitations. Although immunohistochemistry studies are valuable, immunoblotting and cell culture-based studies are more effective for the investigation of molecules at tissue level. For this reason, our results need to be confirmed by these methods. The retrospective design is another limitation. We did not have any information about the patients’ follow-up and HCC occurrence. In addition, our control group was small because it is hard to find liver tissue without inflammation or chronic damage. It is well known that survivin expression is a poor prognostic factor in HCC, but its prognostic role needs to be evaluated in CHB patients. Despite these limitations, we hope that our study will trigger new studies to clarify this issue.