Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus, xerosis, and erythematous papules and plaques [1]. AD is considered a type 2 immune-mediated disease and is often associated with other type 2 diseases like asthma and allergic rhinitis, as well as other conditions such as eosinophilic esophagitis, food allergies, prurigo nodularis and mental health disorders among others [1].
The relationship between AD and the risk of malignancy has been a topic of debate, with conflicting data on this association [2–4]. The increased risk of malignancy in AD patients could be attributed to various factors, including chronic systemic inflammation, and the long-term use of immunosuppressive medications [2–4].
Recently, new advanced and targeted therapies have been approved for the treatment of moderate to severe AD such as dupilumab (IL-4/IL-13), tralokinumab (IL-13 inhibitor), and selective JAK-1 inhibitors such as upadacitinib and abrocitinib. These therapies target specific inflammatory pathways implicated in AD pathogenesis and have shown promising results in clinical trials.
IL-4 and IL-13 have been implicated positively and negatively in tumorigenesis. IL-4 has demonstrated oppositional effects on tumour development while also being implicated in the protection of tumour cells against apoptosis [5]. Similarly, IL-13 has been implicated in enhancing tumour metastasis [6, 7]. On the other hand, the JAK-STAT signalling pathways have shown anti-tumour properties, with STAT1 signalling exhibiting anti-tumorigenic, anti-metastatic, and tumour-suppressing effects while STAT3/STAT5 signalling exhibits tumorigenic, metastatic, and anti-apoptotic effects [8]. As a result, new advanced therapies targeting these molecules and pathways could play a role in enhancing or preventing carcinogenesis. Overall, given the potential risks of treatments, it is essential to understand their effects to ensure the best possible health outcomes. Therefore, this paper examines the risk of malignancy in patients with moderate to severe AD treated with IL-4/13 inhibitors and/or selective JAK-1 inhibitors.
Aim
To gain clarity on the association between biologic and selective JAK-1 therapies and the risk of malignancy in patients with AD, we conducted a systematic review and network meta-analysis (NMA) of randomized controlled trials.
Material and methods
We performed a systematic review and NMA of randomized controlled trials of tralokinumab, dupilumab, abrocitinib, and upadacitinib for the treatment of moderate to severe AD.
Our study protocol was registered with the International Prospective Register of Systematic Reviews (CRD42022355839), and we followed the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA) guidelines [9].
Search strategy
We conducted systematic searches in Ovid MEDLINE and Embase from inception to 23 December 2022.
Eligibility criteria
The eligibility criteria included open label clinical trials, randomized controlled trials, and prospective cohort studies that examined AD patients treated with dupilumab, tralokinumab and selective JAK-1 inhibitors (abrocitinib and upadacitinib). There were no time limitations to this systematic review, however only studies originally in English were included. Studies reporting the incidence of malignancy in AD patients receiving these therapies were included for full-text screening. Ultimately, an article was included in the meta-analysis if it reported: 1) quantitative data on the incidence of malignancy in patients with AD and 2) had both a placebo/control group and an intervention group that included at least one of the eligible therapies (tralokinumab, dupilumab, abrocitinib, or upadacitinib).
Screening
Two reviewers (N.N. and D.M.) independently assessed the eligibility of all titles and abstracts. The most recent available study was included whenever duplicates were found. Full-text screening was performed by the same two reviewers (N.N. and D.M.) and any discrepancies during screening were resolved using a third reviewer (E.S.).
Data extraction
Two reviewers (N.N. and D.M.) independently extracted the data from the eligible studies into a standardized Microsoft Excel file. A third reviewer (E.S.) resolved any discrepancies that arose during data extraction. The extracted data included study design and characteristics (first author, date of publication), number, sex, age, incidence rate of malignancy in control and intervention groups and follow-up period.
Primary outcome
The primary outcome was the incidence of malignancy in patients treated with placebo or one of the advanced therapies mentioned above.
Risk of bias assessment
Risk of bias was assessed independently by two reviewers (D.M. and E.S.) using the Cochrane Risk of Bias Tool [10]. The following six domains were evaluated for having low, some concerns or high risk of bias: risk of bias arising from the randomization process, risk of bias due to deviations from the intended interventions, missing outcome data, risk of bias in measurement of the outcome, risk of bias in the selection of the reported result, and overall risk of bias. Discrepancies were resolved via reviewing studies in consensus.
Data synthesis
We conducted all analyses in Stata (StataCorp., Version 17) [11].
We performed a random-effects NMA using odds ratios (ORs) and 95% confidence intervals (CIs) to assess the association between different treatments and the incidence of malignancy. Our reference comparator was the placebo group.
We conducted random-effects, frequentist NMAs, i.e., multivariate meta-analysis, for the outcome, incidence of malignancy, assuming a common heterogeneity parameter [12, 13] with the mvmeta command [14, 15] and network suite in Stata. We first used the global test of the “design-by-treatment” model to check the coherence assumption in our entire network [16]. We then ran the node-splitting model to assess for incoherence between direct and indirect effect estimates. If incoherence was observed between direct and indirect effect estimates, we used the IF plot to assess loop-specific incoherence [17]. Ranking probabilities for the interventions in our two networks were estimated using the surface under the cumulative ranking curve (SUCRA) values, mean ranks and rankograms [14, 17].
For the purposes of this analysis, different dosages of all agents were classified as unique interventions to evaluate any possible dose-dependent effects. Additionally, we combined arms for multi-arm trials which examined the same intervention, by combining the number of patients and sample sizes across the respective arms.
Results
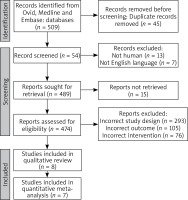
The literature search yielded 474 unique records. After screening titles and abstracts, 25 articles were retrieved for full-text evaluation; and 12 studies met the eligibility criteria. After screening, 11 were included in the analysis as shown in the PRISMA flow diagram (Figure 1).
Study characteristics
The studies included a combined total of 10097 patients with a mean age of 33.2 years and 57.4% male. The mean follow-up period of the studies was 41.4 weeks. Additional details of eligibility criteria and baseline characteristics are presented in Supplement 1.
Quality assessment
Based on the Cochrane Risk of Bias Tool [10], the methodological quality of most of the studies was intermediate to high, with most studies demonstrating either a low risk of bias or some concerns. A final judgment for each paper was then made for overall risk of bias. Discrepancies were resolved via reviewing studies in consensus. Some studies demonstrated a high risk of bias. The prospective cohort study was only included in the systematic review and not the NMA. Details of the quality assessments can be found in Supplement 2.
Incidence of malignancy
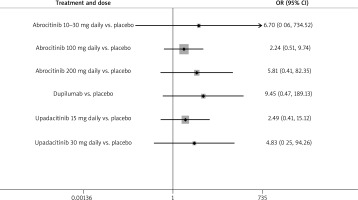
The NMA did not show any statistically significant difference in the incidence of malignancy between selective JAK-1 inhibitors and dupilumab when used to treat moderate to severe AD (Figures 2, 3). For all treatment groups, p-values were greater than 0.05 and the 95% confidence intervals in the forest plot had a lower bound of OR = 0.51 or less, demonstrating a lack of statistical significance (Figure 4). Furthermore, the confidence intervals were large demonstrating increased variability.
Figure 2
Network map showing the associations and their strengths between the various treatment groups, placebo and each other
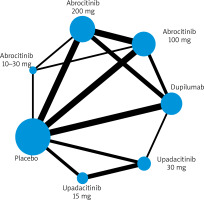
Figure 3
League table showing each treatment group compared to each other, demonstrating no statistically significant difference in incidence rate of malignancy in any treatment groups
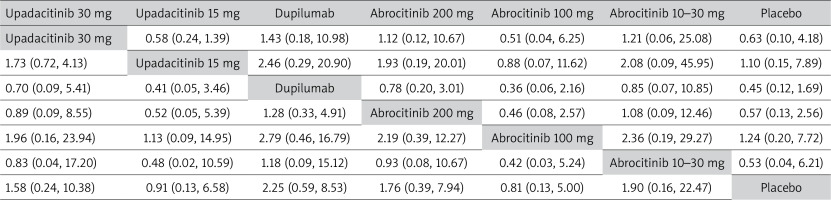
Figure 4
Forest plot with odds ratios showing which of the treatment groups had a statistically significant difference in malignancy incidence when compared to placebo
The network map has been included to demonstrate the comparisons between treatment groups and placebo. In our analysis, comparisons to placebo were the most frequent. Comparisons between different doses of abrocitinib and different doses of upadacitinib, as well as between abrocitinib 200 mg daily and various dupilumab doses, were also conducted (Table 1).
Table 1
Surface under the cumulative ranking (SUCRA) curves showing the probabilities of each treatment being the treatment which causes the least incidence of malignancy
Discussion
We report a NMA examining the risk of malignancy in patients with moderate to severe AD treated with either dupilumab, upadacitinib or abrocitinib including data from 10097 patients in 11 separate trials. The results showed no statistically significant difference in the incidence of malignancy for selective JAK-1 inhibitors and dupilumab compared to placebo, and no significant difference between selective JAK-1 inhibitors and dupilumab when used to treat AD up to an average of 41 weeks of follow-up. This finding contrasts with concerns suggesting an increased risk of malignancy with JAK-1 inhibitors. While clinical observation and case reports have created the foundation for the hypothesis that JAK-1 inhibitors may have an increased risk of malignancy, our NMA has not demonstrated that.
Tofacitinib is a JAK 1,2,3 inhibitor and is approved for the treatment of moderate to severe rheumatoid arthritis (RA), psoriatic arthritis, ulcerative colitis, and juvenile idiopathic arthritis [18]. Tofacitinib has been shown to increase the risk of malignancy in RA patients over a period of 4 years with a hazard ratio of 1.48 when compared to patients treated with TNF-a inhibitors [19].
When considering the safety profile of abrocitinib and upadacitinib for the treatment of AD, it is essential to acknowledge potential variations between patient populations with RA and AD. For instance, individuals with RA may have a higher risk of developing malignancies, including lymphoma and lung cancer, compared to the general population [20]. These differences, along with the selectivity of upadacitinib and abrocitinib for JAK-1, and the relatively shorter mean follow-up period in the studies included in our NMA, may contribute to variations in outcomes between tofacitinib treatment for RA and our study. One other factor is the selectivity of upadacitinib and abrocitinib for JAK-1, unlike tofacitinib which is a JAK1,2,3 inhibitor. Other differences may be related to the shorter mean follow-up period seen in the studies included in our NMA compared to studies on tofacitinib.
Proposed pathophysiological mechanisms for the relationship between various JAK inhibitors and malignancies suggest that the inhibition of specific JAK subtypes might impact natural killer cells’ detection and subsequent elimination of certain malignancies [21]. Extended exposure to pharmacological agents inhibiting the JAK-STAT pathways may lead to prolonged immunologic dysfunction, particularly in natural killer cells, thereby potentially increasing the risk of malignancy in patients.
Malignancies may have a latent clinical presentation, making early detection challenging, and they may not be captured during the relatively short average follow-up period of 41 weeks in the included studies. It is possible that this duration is insufficient for the most commonly associated malignancies with JAK-1 inhibitor use to become clinically evident. Additionally, the doses and durations of JAK-1 inhibitor use in the studies may not have been long enough to induce a pro-inflammatory carcinogenic state that is easily detectable through symptomatic presentation or biomarker screenings. As a result, longer-term observational studies may provide more insights into this outcome than randomized controlled trials.
These results are very clinically pertinent as the belief that JAK-1 inhibitors are carcinogenic when used at specific doses and for certain time periods for AD has significantly influenced and guided clinical decision-making and practice. Our NMA aimed to discern whether these concerns are clinically warranted, and we discovered that at least in the short term (average follow-up of 41 weeks), JAK-1 inhibitors do not significantly increase the risk of malignancies when compared to dupilumab when used for AD.
However, it is crucial to acknowledge the limitations of this NMA. The small sample size (n = 11) restricted the assessment of the transitivity assumption and the examination of substantial heterogeneity observed through sub-group analyses and meta-regression. Despite these limitations, our study achieved coherence in both global and loop-specific instances of the network, highlighting the comparative effectiveness of the included interventions. Additionally, some trials included in our analysis had a high risk of bias, but their consistent results with other studies indicate that their impact on the overall robustness of results is unlikely to be significant. Furthermore, our study could have been enhanced by including other biologic therapies falling within the IL-4/13 inhibitor and JAK-1 inhibitor categories used for AD treatment. Further research may be necessary to explore the incidence of cancer in other non-dupilumab IL-inhibitors and more JAK-1 inhibitors to gain a comprehensive understanding of the global effects of immunomodulatory therapy use for AD.
Conclusions
Based on our analysis, there was no statistically significant increased risk of malignancy and no significant difference in the incidence of malignancy between JAK-1 inhibitors and dupilumab when used for the treatment of AD up to an average follow-up of 41 weeks. These findings have important clinical implications, suggesting that perhaps clinicians should focus more on patient-centered outcomes when selecting advanced therapies for AD. Further research with longer follow-up periods and larger sample sizes may be needed to confirm these results and explore the effects of other biologic therapies in the context of AD.
Materials
Rayyan, also known as rayyan.ai, was used to conduct and complete the screening [22].
Google Sheets & Microsoft Excel were used to conduct extraction of all included papers.
Google Docs & Microsoft Word were used for the drafting and editing of the manuscript.
Stata was used to conduct the network meta-analysis and any other relevant statistical analyses [11].
The Prisma flow diagram and Prisma checklist was retrieved from http://prisma-statement.org/ [9].
Zotero was used to facilitate the citation process.
CINeMA was used to assess confidence in the NMA results [23].