Introduction
Platelet-rich-plasma (PRP) is defined as autologous plasma, rich in growth factors, platelet-rich fibrin (PRF) matrix, and platelets [1]. Platelets are anucleate biconvex discoid cell fragments 2–3 µm in diameter containing various cellular receptors on their surface, enabling binding with multiple factors, such as von Willebrand factor, thrombin, and fibrinogen. Due to these abilities platelets are the first responders to a wound/tissue repair and play a critical role in wound healing mechanism [2, 3]. Nowadays PRP is one of the most commonly used preparations in regenerative medicine because it contains a high concentration of growth factors and cytokines that participate in various cellular, immune, and regenerative processes, such as wound healing and tissue regeneration [4]. Specific growth factors and cytokines in PRP include, i.a.: transforming growth factor-β, fibroblast growth factor, platelet derived growth factor, insulin-like growth factors 1 and 2, vascular endothelial growth factor, and epidermal growth factor [4]. Platelet-rich-plasma is widely used in orthopaedics, dermatology, plastic surgery, cardiothoracic surgery, dentistry, and diabetic wound healing [5–8].
Postoperative wound dehiscence after laparotomy is a serious complication that leads to higher mortality rates, increased readmissions and social costs [9]. Previous research has identified a number of risk factors for this complication. These factors can be divided into 4 groups:
Patient-related factors – such as: smoking, obesity, diabetes, and the use of immunosuppressive agents;
Procedure-related factors – such as: operation type, type of incision and closure, and duration of surgery;
Postoperative factors – such as clean wound classification, coughing, and wound infection;
Operative parameters – e.g. qualifications of the surgeon, emergent surgery [9, 10].
Oncological patients, regardless of type of cancer, are at high risk of developing postoperative wound dehiscence [11].
The aim of this study was to evaluate if PRP application into the wound during surgery in gynaecological cancer patients improves wound healing and reduces pain in the postoperative period.
Material and methods
In this single-blind placebo-controlled intervention study, adult women undergoing surgical treatment because of female genital tract malignancies in the Department of Obstetrics, Women’s Diseases, and Oncogynaecology, National Medical Institute of the Ministry of the Interior and Administration in Warsaw, Poland between January 2018 and May 2019 were included. The inclusion criteria included the following: age > 18 years and diagnosis (or suspicion) of gynaecological malignancy (ovarian, endometrial, or cervical cancer) with the qualification for surgical treatment by laparotomy. The exclusion criteria included the following: allergy for analgesics, viral or bacterial local infections, coagulation disorders, body mass index > 40 kg/m2, and lack of consent for enrolment in the study. Patients were randomly assigned to one of 2 groups: group 1 – patients who received the application of PRP into the wound during the surgery, and group 2 (control group) – patients who received the application of placebo (0.9% NaCl solution). The allocation ratio was 1:1. The randomisation was performed manually, and the allocation concealment was performed by using sequentially numbered opaque envelopes. The study was single-blind, meaning the participants were unaware of the treatment they received.
The primary outcome was the wound dehiscence, whereas the secondary outcomes were postoperative pain intensity, the use of analgesics, scar quality assessment, and quality of life assessment after surgery. Wound dehiscence was defined as both complete and partial wound separation (> 1 cm long) diagnosed by the clinician during the follow-up period. The study was approved by the Bioethical Committee of Central Clinical Hospital of Interior in Warsaw (No. 99/2016, approval date: 09.11.2016), and informed consent was obtained from all patients. The study was registered in the IRSCTN registry with registration number ISRCTN17395989. The sample size of participants (23 treatment participants, 23 control participants) was estimated by power analysis to achieve greater than 80% power to detect a 35% change in the incidence of wound dehiscence.
Surgical treatment was performed by laparotomy with the midline incision in all patients. At the end of the surgery, during the abdominal closure, PRP or placebo (0.9% NaCl solution) was applicated by a series of microinjections into the abdominal muscle fascia and subcutaneous tissue. All operations were performed by the same surgeon, an experienced specialist in gynaecological oncology. All patients received antibiotic prophylaxis with a single dose of 2 g cefazolin administered intravenously up to 20 minutes prior to skin incision. During the surgery all patients had general anaesthesia, standardised according to the local protocols used in the hospital. Patients did not receive any additional type of anaesthesia (including epidural). The basic postoperative analgesic therapy was intravenous paracetamol and morphine administered in the form of patient-controlled analgesia (PCA). Additionally, some patients were treated with intravenous metamizole or ketoprofen, when basic treatment was insufficient. To avoid bias, patients with allergy for analgesics were excluded from the study. Each patient was asked to evaluate the pain by using a visual analogue scale (VAS) immediately after the surgery and also 6 and 12 hours after the surgery. The use of analgesics was recorded as the mean and total morphine boluses needed, as well as the number of doses of paracetamol, metamizole, and ketoprofen needed per day.
The appearance of the postoperative scar was assessed using the patient and observer scar assessment scale (POSAS), a reliable and valid scar assessment scale that measures scar quality from 2 perspectives: the patient and the clinician (the observer). It includes the assessment of 6 parameters: vascularity, pigmentation, thickness, relief, pliability, and surface area. Each parameter can be scored on a 1-10 scale, where the lowest score of “1” indicates normal skin and the highest score of “10” indicates the worst scar imaginable. In the presented study POSAS was recorded on days 1, 8, 30, and 90 after surgery.
Quality of life assessment was performed using the SF-12 questionnaire, including 8 life domains. All patients were asked to fill out the questionnaire on days 1, 8, 30, and 90 after surgery.
Platelet-rich-plasma preparation
The blood of an individual was collected by venipuncture in a sterile tube with a special gel – chemical polymer enabling efficient separation of morphotic elements from plasma (Regeneris®, Regen Lab SA, Le Mont-sur-Lausanne, Switzerland). This was followed by centrifugation for 5 min at 1500 × g and PRP was present above the separating gel and platelet activating factor (thrombin) was added. Then the PRP was ready to use during the surgery. In this protocol about 8–10 ml of PRP was derived 24–30 ml of each patient’s whole blood.
Statistical analysis
The basic characteristic of study participants are presented by descriptive statistics. Because the sample size was relatively small (< 30/group):
The Mann-Whitney U test to assess the significance level was used for continuous variables;
The χ2 test was used for categorised data if the assumption of the expected values no less than 5 was fulfilled, otherwise the Fisher’s exact test was used;
For wound dehiscence, binomial logistic regression with bootstrap analysis including 20,000 repetitions was implemented;
To assess the impact on pain, an ordinal logistic regression with bootstrap analysis (20.000 repetitions) was run.
Ordinal regression provided a risk estimate to observe a patient with a pain VAS-score higher by 1, meaning the dependent variable is a scale with ordered response options of 1–10, the dependent is intervention (PRP/placebo), the model calculates a risk of reporting more intense (by 1 point in VAS scale) pain associated with PRP, e.g. to observe 5 points (VAS-score) instead of 4 points, or to observe 9 instead of 8. The overall ratio < 1 reflects decreased risk of observing higher pain intensity. Analyses were performed using Stata 13.1, StataCorp LP, TX, USA. Results with the p-value less than 0.05 were considered statistically significant.
Results
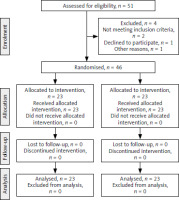
Forty-six patients were included in the study: 23 women in the PRP group and 23 in the placebo group. The consolidated standards of reporting trials flowchart for patient recruitment and analysis is presented in Figure 1. The demographic and clinical characteristics of the study participants are summarised in Table 1.
Table 1
Demographic and clinical characteristics of the study participants
Wound dehiscence was diagnosed in 5 (10.8%) patients: 1 (4.3%) patient in the PRP group and 4 (17.4%) women in the control group; however, this difference did not reach statistical significance (p = 0.346). However, after binominal logistic regression with bootstrap analysis in both univariable and multivariable models, the risk of developing wound dehiscence after PRP application was significantly lower in comparison to the control group (Table 2).
Table 2
Binominal logistic regression with bootstrap analysis for the risk of wound dehiscence in patients treated with platelet- rich-plasma
| Parameters | OR | Normal-based 95% CI | p-value |
|---|---|---|---|
| Univariable model | 0.22 | 0.048–0.97 | 0.046 |
| Multivariable model* | 0.17 | 0.03–0.92 | 0.040 |
There was a difference in the pain intensity assessment in VAS scale recorded 12 hours after surgery – PRP patients had significantly lower mean VAS score at this moment (3.83 ±1.19 vs. 4.7 ±0.88; p = 0.014). The visual analogue scale scores recorded immediately after surgery, as well as 6 hours after surgery, were similar in both groups. Additionally, PRP patients required fewer total morphine doses than the control group (8.22 ±3.3 vs. 10.96 ±5.05, respectively; p = 0.048). There was no difference between the groups in the use of paracetamol and metamizole; however, there was a trend of fewer doses of ketoprofen needed per day in the PRP group than in the control group (0.26 ±0.45 vs. 0.7 ±0.82, respectively; p = 0.062). Ordered logistic regression analysis confirmed, in both univariable and multivariable models, significantly lower VAS scores 12 hours after surgery in PRP patients in comparison to the placebo group (Table 3).
Table 3
Ordered logistic regression for the risk of reporting more intense (by 1 point in visual analogue scale scale) pain associated with treatment with platelet-rich-plasma
Significant differences between the groups in the scar quality assessment were also detected in both the patient and doctor POSAS on days 8, 30, and 90 after surgery. These results are presented in detail in Tables 4, 5. Evaluation of the SF-12 questionnaire did not reveal any differences between the groups, indicating that the quality of life was similar in both groups on every recorded day after surgery.
Table 4
Results of scar quality assessment (patient and observer scar assessment scale) by patients in the study groups (presented as mean ±SD)
Table 5
Results of scar quality assessment (patient and observer scar assessment scale) by clinicians in the study groups (presented as mean ±SD)
Discussion
The present study aimed at assessing the usefulness of PRP application during laparotomy in improving wound healing and reducing postoperative pain intensity. Although the incidence of wound dehiscence did not differ significantly between the groups, logistic regression analysis demonstrated that PRP application was related to the significantly lower risk of developing wound dehiscence in comparison to the control group. Additionally, the risk of reporting more intense pain associated with treatment with PRP 12 hours after surgery was significantly reduced and the use of analgesics in the postoperative period was also lower after PRP application in comparison to the control group. Scar quality was also significantly better in both short- and long-term assessment in women treated with PRP in comparison to placebo.
Wound healing is a process that results in the restoration of normal architecture and function of damaged tissue through a physiological process, which is divided into 4 phases: haemostasis, inflammation, proliferation, and remodelling [12, 13]. Platelet-rich-plasma action in wound healing is based on stimulating the synthesis of matrix metalloproteinases, increasing cutaneous fibroblast growth, as well as the production of extracellular matrix components including type I collagen and elastin [14–16].
The incidence of postoperative wound dehiscence after laparotomy is estimated in available literature at 1–31%, depending on multiple factors; however, it is highest in patients operated on in medical emergencies, and in patients with malignant disease [17]. A Norwegian study, based on the administrative data from all Norwegian hospitals for the period 2011–2015, demonstrated that the overall rate of postoperative wound dehiscence was 1.89%, but it varied between hospitals (0–5.1%) [9]. In a recently published meta-analysis including 741,118 patients across 24 studies, the incidence of wound dehiscence was very low (1%), but it referred not only to laparotomy but also to laparoscopy [18]. There is little research concerning wound complications in gynaecological malignancies. Nhokaew et al. revealed 7.8% incidence of wound complications after laparotomy for endometrial cancer, whereas other studies demonstrated this rate as 25.1–33.9% [10, 19, 20]. It is similar in ovarian cancer [21, 22]. In our study the overall incidence of wound dehiscence was 10.8%, but in the group treated with PRP it was only 4.3%. The relatively high incidence of wound dehiscence in our study is related to the definition of this complications we adopted in the study – both complete and partial dehiscence (> 1 cm long). The differences in the prevalence of wound dehiscence between different studies results from completely different definitions of this complication. In research based on national registries or hospital administrative data, wound dehiscence is defined as the wound separation requiring reoperation, whereas in other studies it is any separation diagnosed by the surgeon or physician in the postoperative period [9, 10, 23]. It needs to be highlighted that postoperative wound dehiscence is a serious complication with mortality rate of up to 44%; therefore, any intervention to avoid this condition is of great value [24, 25].
There is a vast body of evidence for the role of PRP in improving wound healing in various situations, such as foot ulcers associated with diabetes, orthopaedics, and sports medicine [6, 26, 27]. In gynaecology, Morelli et al. conducted a study on 25 women who had undergone radical surgery because of vulvar cancer. The application of platelet-rich gel was related to a significant reduction in wound infection, necrosis of vaginal wounds, and wound breakdown rates [28]. Another study of 55 patients undergoing major gynaecological surgery demonstrated that autologous platelet grafts were effective for pain reduction and were not associated with any adverse effects [29]. In our previously published study PRP application during caesarean section significantly improved wound healing in both short- and long-term assessment [30]. In presented study the application of PRP during surgery also had a positive effect on wound healing by reducing the risk of wound dehiscence and improving results of scar quality assessment. It is of great value in oncological patients, who are at high risk of wound healing complications, and additionally, in 5–10% of them, chemo- or radiotherapy predisposes to development of ulcer wounds and fungal infections because of disturbances in tissue vascularisation [31–33]. There are a broad range of factors that may affect wound healing in oncological patients, including comorbidities and adjuvant therapies. On the other hand, oncological patients significantly more often suffer from chronic diseases, such as diabetes or hypertension. Women with genital tract malignancies, especially with endometrial cancer, often have a high burden of comorbidities that significantly affect their survival outcomes. In our study there was no difference between the groups in the incidence of comorbidities, including diabetes. Unfortunately, we do not have any information about adjuvant treatment, such as chemo- or radiotherapy, that patients could receive after surgery, and we could not take it into consideration. There is a need for a correct and complete evaluation of patients with gynaecological malignancies, considering i.a. various comorbidities and older age, to identify those at highest risk of complications, and to implement tailored preventive and therapies [34, 35].
An additional effect of PRP use during surgery is postoperative pain reduction and decreased use of analgesics. Inappropriate treatment of postoperative pain can contribute to delays in the patient’s recovery, the development of chronic postoperative pain, an increased incidence of pulmonary and cardiac complications, as well as increased morbidity and mortality [36, 37]. Among various surgical procedures gynaecology together with orthopaedics, abdominal surgery and cardiothoracic surgery are among the procedures rated worst by patients in terms of postoperative pain [36]. For a long time, opioids have served as the drugs of choice in the treatment of post-operative pain. However, with the recent awareness of the opioid epidemic in the United States any intervention that can diminish the use of opioids should be considered in clinical practice [38]. Guidelines for enhanced recovery after surgery in gynaecology and gynaecological oncology, updated in 2019, also pay attention to a modal post-operative opioid sparing analgesic protocol [39]. Our study demonstrated that the use of PRP during surgery in gynaecological oncological women significantly decrease the use of morphine in the early postoperative period.
The main limitation of this study is the small number of cases. Additionally, the study group consisted of patients with different malignancies (ovarian, cervical, and endometrial cancer). However, the type of abdomen opening and closure was exactly the same in all patients. Other limitations are also the lack of information about adjuvant therapy (chemo- or radiotherapy) that patients could receive after surgery (it could be important for long-term scar-quality assessment) and about the length of skin incision. The strength of the study is the use of the POSAS scale, evaluated by both the patient and clinician, making the assessment more objective. Similarly, postoperative pain feeling was evaluated not only by VAS scale, but also with the use of analgesics, making it more reliable as well. To the best of our knowledge, this is the first study comparing the use of PRP with placebo during gynaecological oncology procedures, indicating that this group of women may benefit from PRP use.
Conclusions
The presented study demonstrated that PRP application during abdominal closure in patients with gynaecological malignancies undergoing laparotomy improves wound healing and also reduces pain and the use of analgesics in the early postoperative period. Taking into consideration that cancer patients are at high risk of wound complications that may have detrimental effects for further treatment, i.a. causing a need to postpone adjuvant treatment, such as chemo- or radiotherapy, the use of PRP may be a promising and completely safe method for enhanced recovery in this group of women.