Atopic dermatitis (AD) is a chronic inflammatory skin disease. Multiple immune cells and cytokines participate in the genesis of AD, such as Th2 cells, dendritic cells and interleukin (IL) 4 (IL-4), IL-5, IL-13, and IL-31 [1]. Abrocitinib is a selective JAK1 inhibitor that exerts its therapeutic effects by inhibiting downstream effects of the JAK-STAT pathway, suppressing the production of cytokines, such as IL-4, IL-13, and IL-31 [2, 3]. Clinical studies have shown efficacy of abrocitinib, while it also has some potential adverse effects, such as infections, and thrombocytopenia [3, 4]. In this study, we evaluated the changes of platelet counts after abrocitinib treatment through meta-analysis.
Publications were selected from six databases (Supplementary Figures S1 and S2, Table 1). The standardized mean difference (SMD) was used to assess the trends, aiming to eliminate the effects of differences in measurement units and methods. According to the between-study heterogeneity, the fixed or random effect model was applied. For each included study, the Newcastle-Ottawa scale (NOS) or the Cochrane bias evaluation tool was used to assess the study quality based on the study type.
Table 1
Characteristics of included studies
| Study ID | PMID | Type | Quality | Countury | Severity | Age | Dose | Other treatment | Unit | Before treatment | After treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Level | Week | N | Level | ||||||||||
| Gooderham 2019 | 31577341 | RCT | High | International | Moderate to severe | 41.1 ±15.6 | 100 mg qd | Oral antihistamines were permitted | 109/l | 56 | 279.75 ±110.17 | 1 | 56 | 279.75 ±112.59 |
| 2 | 56 | 257.25 ±104.54 | ||||||||||||
| 4 | 56 | 244.75 ±98.91 | ||||||||||||
| 6 | 56 | 254.25 ±101.33 | ||||||||||||
| 8 | 56 | 257.25 ±115.00 | ||||||||||||
| 12 | 56 | 269.00 ±131.08 | ||||||||||||
| 38.7 ±17.6 | 200 mg qd | Oral antihistamines were permitted | 109/l | 55 | 270.75 ±78.46 | 1 | 55 | 257.00 ±89.67 | ||||||
| 2 | 55 | 223.25 ±90.47 | ||||||||||||
| 4 | 55 | 189.25 ±96.07 | ||||||||||||
| 6 | 55 | 216.75 ±94.47 | ||||||||||||
| 8 | 55 | 216.25 ±89.67 | ||||||||||||
| 12 | 55 | 238.75 ±120.09 | ||||||||||||
| Simpson 2020 | 32711801 | RCT | High | International | Moderate to severe | 32.6 ±15.4 | 100 mg qd | Oral antihistamines were permitted | 103/mm3 | 156 | 285.10 ±62.91 | 2 | 156 | 251.44 ±59.77 |
| 4 | 156 | 241.32 ±59.77 | ||||||||||||
| 8 | 156 | 244.27 ±68.41 | ||||||||||||
| 12 | 156 | 255.06 ±63.76 | ||||||||||||
| 33.0 ±17.4 | 200 mg qd | Oral antihistamines were permitted | 103/mm3 | 154 | 282.41 ±71.74 | 2 | 154 | 224.62 ±66.42 | ||||||
| 4 | 154 | 204.47 ±59.78 | ||||||||||||
| 8 | 154 | 228.46 ±62.44 | ||||||||||||
| 12 | 154 | 242.68 ±64.43 | ||||||||||||
| Bieber 2021 | 33761207 | RCT | High | International | Moderate to severe | 37.3 ±14.8 | 100 mg qd | Topical therapies were allowed | 103/mm3 | 238 | 280.20 ±64.64 | 2 | 226 | 253.90 ±63.14 |
| 4 | 228 | 231.90 ±61.91 | ||||||||||||
| 8 | 219 | 246.30 ±64.97 | ||||||||||||
| 12 | 221 | 257.90 ±68.09 | ||||||||||||
| 16 | 215 | 261.10 ±65.10 | ||||||||||||
| 38.8 ±14.5 | 200 mg qd | Topical therapies were allowed | 103/mm3 | 226 | 277.60 ±66.90 | 2 | 220 | 231.90 ±61.26 | ||||||
| 4 | 221 | 201.50 ±57.98 | ||||||||||||
| 8 | 216 | 220.10 ±56.14 | ||||||||||||
| 12 | 207 | 234.20 ±54.67 | ||||||||||||
| 16 | 203 | 241.50 ±58.27 | ||||||||||||
| Blauvelt 2021 | 34416294 | RCT | High | International | Moderate to severe | 29.0 [20.0, 41.0] | 200 mg qd | — | 103/mm3 | 798 | 280.23 ±65.50 | 2 | 765 | 220.65 ±60.50 |
| 4 | 779 | 192.88 ±54.24 | ||||||||||||
| 8 | 786 | 216.60 ±59.25 | ||||||||||||
| 12 | 773 | 223.51 ±58.41 | ||||||||||||
| Eichenfield 2021 | 34406366 | RCT | High | International | Moderate to severe | 16.0 [14.0, 17.0] | 100 mg qd | Topical therapy; oral antihistamines were permitted | 103/µl | 95 | 293.56 ±52.50 | 2 | 95 | 265.80 ±66.40 |
| 4 | 95 | 250.00 ±77.21 | ||||||||||||
| 8 | 95 | 260.74 ±77.21 | ||||||||||||
| 12 | 95 | 268.00 ±63.31 | ||||||||||||
| 15.0 [13.0, 16.0] | 200 mg qd | Topical therapy; oral antihistamines were permitted | 103/µl | 94 | *302.75 ±71.03 | 2 | 94 | 254.59 ±77.20 | ||||||
| 4 | 94 | 227.36 ±75.68 | ||||||||||||
| 8 | 94 | 253.52 ±71.03 | ||||||||||||
| 12 | 94 | 265.45 ±61.77 | ||||||||||||
| Silverberg 2022 | 32492087 | RCT | High | International | Moderate to severe | 37.4 ±15.8 | 100 mg qd | Oral antihistamines were permitted | 103/mm3 | 158 | 267.85 ±63.78 | 2 | 158 | 235.27 ±57.32 |
| 4 | 158 | 216.49 ±58.94 | ||||||||||||
| 8 | 158 | 227.51 ±65.40 | ||||||||||||
| 12 | 158 | 233.02 ±68.63 | ||||||||||||
| 33.5 ±14.7 | 200 mg qd | Oral antihistamines were permitted | 103/mm3 | 155 | 252.97 ±65.41 | 2 | 155 | 218.24 ±70.26 | ||||||
| 4 | 155 | 191.84 ±58.14 | ||||||||||||
| 8 | 155 | 210.83 ±54.91 | ||||||||||||
| 12 | 155 | 219.26 ±61.37 | ||||||||||||
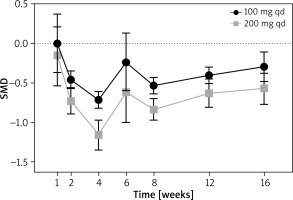
A total of six Randomized Controlled Trials (RCT) were included (Supplementary Figure S3 shows the criteria). All the studies were considered as high-quality, because “low risk” and “unclear risk” were rated through the Cochrane bias evaluation tool. All the included studies reported the dose of 200 mg qd, while five reported 100 mg qd. The baseline levels of platelet counts were all considered as normal besides the 200 mg qd group in the study of Eichenfield 2021 (Table 1), and after treatment, all the counts remained normal. When abrocitinib was used with 100 mg qd, the numbers of platelets were decreased significantly at week 2, 4, 8, 12 and 16, with SMD = –0.46 (95% CI: –0.57, –0.35); –0.72 (95% CI: –0.82, –0.61); –0.54 (95% CI: –0.64, –0.43); –0.40 (95% CI: –0.51, –0.30), respectively. At week 1 and 6, no significant changes were found. When abrocitinib was used with 200 mg qd, the numbers of platelets did not show significant change at week 1. Then, at week 2, 4, 6, 8, 12 and 16, significant decreases were shown, with SMD = –0.73 (95% CI: –0.89, –0.56); –1.16 (95% CI: –1.35, –0.97); –0.62 (95% CI: –1.00, –0.23); –0.84 (95% CI: –0.97, –0.70); –0.63 (95% CI: –0.81, –0.45); –0.57 (95% CI: –0.77, –0.38), respectively (Figure 1, and Supplementary Figure S4).
We summarized the platelet count change trends over different weeks for both dose groups. For each dose group, the lowest point occurred at week 4, and as the treatment duration extended, the number of platelets showed an increasing trend. Compared to the 100 mg once daily dose group, patients receiving the 200 mg once daily treatment exhibited a more significant reduction. From the funnel plots, for both dose groups, the diagram showed asymmetry, which indicated that the results may not robust perfectly (Supplementary Figures S5).
Our study found that abrocitinib can cause a decrease in platelet counts in AD patients after treatment, with the lowest point at week 4 and then showing a tendency towards normal levels. Furthermore, we found that the dose of 200 mg qd had a more pronounced effect than the 100 mg qd, and this was similar to that observed in other RCTs [3]. The mechanism is not clear, yet may be mediated by the inhibition of JAK1 or through the inhibition of the Ashwell-Morell receptor and downstream effects on platelet production [5]. The limitations of our study: a relatively short period was observed, with at most 16 weeks; and in each comparison, the number of studies included was small, so no subgroup analysis was performed. Further well-designed studies with larger samples, focusing on a long-term treatment are needed.
In clinical practice, more attention should be paid to platelet-related conditions for patients receiving abrocitinib treatment. Furthermore, future research should be conducted to assess the applicability of abrocitinib in patients with platelet dysfunction.