INTRODUCTION
Approximately 30-50% of cancer patients, particularly those with advanced cancer, will experience pain, which tends to increase as the disease progresses [1]. Epidemiological studies, such as those conducted by Ryan et al. [2], indicate that the most common cancers that metastasize to the bones are lung, prostate, and breast cancers. Other cancers that metastasize to the bones include those of the kidney, pancreas, bladder, colorectal, stomach, esophagus, liver, melanoma, as well as cancers of the endocrine system, oral cavity and pharynx, and soft tissue.
Skeletal-related events (SREs) are a common complication of bone metastases leading to severe pain, increased mortality risk, higher medication costs, and reduced quality of life. When cancer spreads to the bones, it disrupts the natural balance between bone formation by osteoblasts and bone destruction by osteoclasts, which can lead to various complications, including hypercalcemia caused by malignancy, bone marrow dysfunction and, more commonly, SREs.
SREs include spinal cord compression, pathological fractures, and the need for bone radiation or surgery. These events can cause severe pain and impose significant physical and financial burdens. Pain associated with bone cancer typically originates from areas densely populated with nerve endings, such as the periosteum, spinal cord, and cortical bone, with a nerve fiber ratio of 100 : 2 : 0.1. The periosteum, which has sympathetic innervation and a high density of sensory fibers, including A-δ fibers and C fibers, plays a crucial role in this pain [3, 4]. The pain is often described as dull and persistent but can intensify as bone remodeling progresses.
When assessing the impact on quality of life, including physical and emotional well-being and functionality, patients frequently report significant declines following their first SREs. It is challenging to study pain associated with SREs even though data indicate that SREs are associated with considerable bone pain.
Bisphosphonate agents and denosumab are therapeutic options for preventing SREs in advanced cancer patients with bone metastases. Notably, fewer patients who received intravenous bisphosphonate therapy (zoledronic acid [ZA]) or subcutaneous denosumab to prevent SREs reported experiencing clinically significant pain compared to those who received a placebo. Effective pain management associated with SREs is crucial for preserving mobility, physical independence, and achieving successful treatment outcomes [5-7]. However, the efficacy of the two treatments in preventing pain remains unclear.
This study aims to compare the efficacy of denosumab and ZA in managing SREs, with a particular focus on pain-related SREs.
METHODS
Search strategy
This study was conducted from January to March 2023, using Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines and schema. A thorough literature search was conducted utilizing electronic databases including Pubmed, the Cochrane Library, and Google Scholar. No restrictions were imposed on the publication date. Keywords were chosen based on the patient, intervention, comparison, and outcome (PICO) framework, as outlined in Table 1. The keyword combinations used were: (1) “neoplasm” OR “cancer” OR “tumor”, and (2) “denosumab” AND “zoledronic acid” and (3) “skeletal-related event” OR “pain”.
Table 1
Eligibility criteria
Inclusion and exclusion criteria
In this study, the inclusion criteria encompassed research published in English between 2010 and 2023, focusing on randomized clinical trials with complete manuscripts available. Articles in the form of case reports, case-control studies, descriptive studies, cross-sectional studies, and cohort studies were excluded. Titles and abstracts were manually reviewed to determine alignment with the eligibility criteria (Table 1).
Study selection and data extraction
The literature selection process was conducted in two stages. The first involved screening for titles and abstracts by each of the two authors to sort relevant articles. The full text of the selected articles was evaluated for compliance with the eligibility criteria (Table 1) by both authors. Consent of both authors was required at the two stages of the process. A third reviewer resolved any differences of opinion.
Data extracted from each study included characteristics of the study (design, follow-up, sample size), type of intervention, and patient outcomes (Table 2). The primary outcome assessed was the delayed first incident of SREs, with secondary outcomes including delayed pain worsening and side effects. Data extraction was arranged in a separate template, tested in several studies, and modified as needed. I.P.E.W. extracted data, and C.T. verified the extracted data. Differences of opinion were resolved through discussion among the authors.
Table 2
Characteristics of included studies
| Factor | Henry et al., 2014 [10] | Stopeck et al., 2010 [5] | Lipton et al., 2012 [11] | Cleeland et al., 2013 [12] |
|---|---|---|---|---|
| Cancer type | Various solid tumors (except breast/prostate cancer), multiple myeloma | Breast cancer | Breast cancer, prostate cancer, other solid tumors, or multiple myeloma | Advanced breast cancer |
| Intervention | Denosumab 120 mg SC vs. ZA 4 mg IV | Denosumab 120 mg SC vs. ZA 4 mg IV | Denosumab 120 mg SC vs. ZA 4 mg IV | Denosumab 120 mg SC vs. ZA 4 mg IV |
| Follow up time | 34 months | 17 months | 24 months | 34 months |
| Sample size | 800/797 | 1026/1020 | 2862/2861 | 542/500 |
| Primary outcome (delayed first incident of SRE) | RR = 0.81; 95 % CI: 0.68-0.96; p = 0.017 | HR = 0.82; 95% CI: 0.71-0.95; p = 0.01 | HR = 0.83; 95% CI: 0.76-0.90; p < 0.001 | – |
| Secondary outcome (delayed pain worsening) | HR = 0.83; 95% CI: 0.71-0.97; p = 0.016 | – | – | HR = 0.78; 95% CI: 0.67-0.92; p = 0.0024 |
| Side effects of denosumab | Grade 3 or 4 hypocalcemia, osteonecrosis of the jaw | Hypocalcemia, osteonecrosis of the jaw | Hypocalcemia | – |
Quality assessment
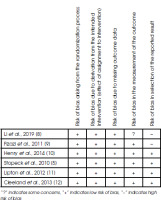
The Cochrane Collaboration Risk of Bias Tool was used to assess the quality of each selected study. Five adjusted dimensions of this tool were used, categorizing the risk of bias as low, high, or some concerns. P.T. and A.V. independently assessed the methodological quality of each study. Differences of opinion were resolved through discussion among all authors.
RESULTS
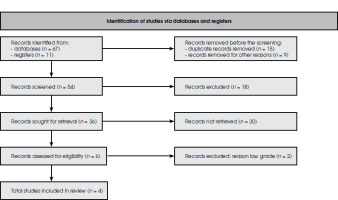
A total of 78 studies were identified during initial research. After removing duplicates and abstracts, 54 studies remained to be assessed. An additional 18 studies were removed after screening the titles and abstracts and applying inclusion and exclusion criteria (Figure I). Of 36 articles, 30 were excluded due to the unavailability of reports, and the remaining six articles were assessed for eligibility. Among these six studies, two failed to meet the requirements. Li et al. [8], and Fizazi et al. [9], did not explain the details of the reported result, and the method was not clear enough. Four randomized clinical trials (Figure II) were further investigated. Patients with pain were compared for the two treatments.
Four RCT studies (Table 2) were selected, which were investigated further. In the four studies, the intervention involved administering 120 mg denosumab subcutaneously every four weeks and compared with giving a dose of 4 mg ZA intravenously every four weeks, with follow-up periods ranging from 17 months in the trial conducted by Stopeck et al. [5], to 34 months in the trial by Henry et al. [10]. The largest sample size was found in the trial by Lipton et al. [11], with 2862 receiving denosumab, and 2861 receiving ZA.
Based on the study by Henry et al. [10], denosumab 120 mg SC prolonged the first incident of SRE by 0.85 times compared to ZA 4 mg IV. Similar findings were reported by Stopeck et al. [5], and Lipton et al. [11]. For delayed pain worsening, Henry et al. [10], and Cleeland et al. [12], found that denosumab administration delayed pain worsening by 17-22% compared to ZA, with median times 5.6-9.7 months (denosumab) vs. 4.6-5.8 months (ZA). In terms of safety, hypocalcemia and jaw osteonecrosis were more common in the denosumab treatment group in three studies.
DISCUSSION
Denosumab therapy is more effective in preventing bone-related complications compared to ZA. This systematic review found that denosumab was superior in delaying the first incident of SRE compared to ZA, which is consistent with previous reviews. This is attributed to the different mechanisms of denosumab and ZA in inhibiting osteoclast activity. ZA inhibits osteoclast function and survival, while denosumab, as a monoclonal antibody, binds to and neutralizes the receptor activator of nuclear factor kappa-B ligand (RANK-L), supporting the RANK-L pathway as crucial in SREs [13, 14].
Pain in bone cancer metastases is multifactorial, including allogenic biologic agents in the metastatic area, increased pressure on bones and nerves due to the growth of metastases in the bone stretching the periosteum, microfractures in bone trabecula, pathological fractures that decrease bone integrity due to tumor growth, infiltration of tumor cells into the nerve roots, and tumor cells that directly damage sensory nerve fibers in the bone [15, 16]. Reactive muscle spasms and increased calcium concentration also cause bone cancer pain. Prolonged cancer pain activates the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic central nervous system causing psychological stress linked to a feedback effect activating the HPA axis further, leading to immune dysregulation which promotes cancer cell growth and neuroendocrine activity. All of these mechanisms are factors in the occurrence of cancer pain. The key pathway to bone pain in other cancer-related conditions is the increased osteoclast activity triggered by RANK-L leading to increased bone resorption and acidification of the bone microenvironment, triggering nerve pain transmitters [1, 4].
Until this point, the treatment of bone cancer pain has been plagued by unforeseen side effects. This is why ongoing research is focused on searching for therapies that can effectively alleviate bone cancer pain- denosumab being one such option. Three clinical trials reported that denosumab was more effective in preventing the progression of pain compared to ZA (HR 0.83; 95% CI: 0.76-0.92; p < 0.001). Denosumab was also shown to be more effective in slowing the worsening of pain compared to ZA in this study, which included more recent trials. Denosumab’s superior effect is believed to stem from its ability to inhibit osteoclast formation and activity to a greater extent through RANK-L inhibition compared to ZA, suppressing bone damage triggered by cancer cells. Compared to ZA, denosumab’s greater effectiveness in delaying SREs indirectly contributes to better pain prevention. However, denosumab and ZA are not analgesics and cannot be expected to reduce pre-existing pain [11, 12].
ZA’s most significant adverse effect, unlike denosumab’s, is potential kidney damage, a crucial distinction as kidney problems are common in cancer patients and tend to worsen with disease progression. Kidney dysfunction is linked to diminished treatment options, quality of life, and survival. Patients treated with denosumab experienced fewer kidney-related adverse events compared to those receiving ZA, including reduced occurrences of elevated creatinine levels and acute phase reactions. While hypocalcemia was more common in the denosumab group, most occurrences were mild to moderate (grade 1-2) [5, 9, 11, 17]. This study observed a reduced occurrence of elevated creatinine levels above 2 mg/dl and fewer instances of a double increase of creatinine levels during the trial. ZA is known to trigger acute phase reactions, which can impact treatment tolerability and add to the patient’s burden. In this study, the denosumab treatment group experienced fewer acute phase reaction events, especially those related to fever and joint pain [18-20]. While hypocalcemia was found to be more common in the denosumab group compared to the ZA group, the majority of these occurrences were of mild to moderate severity (grade 1-2) [5, 9, 11, 17]. Importantly, all studies confirmed similar survival rates between denosumab and ZA treatment groups [15, 16].
This systematic review has limitations, such as a small number of studies meeting the inclusion criteria. However, all selected studies were RCTs with a low risk of bias risk and used the same dosage of denosumab and ZA. Overall, denosumab successfully achieved the primary endpoint of the study and demonstrated non- inferiority to ZA in preventing SREs and related pain. The results of this systematic review support the use of denosumab as an additional treatment option alongside standard management for patients at risk of SREs and associated pain. Nevertheless, larger multicenter clinical trials are needed to assess the efficacy of denosumab in managing bone metastasis-related pain and the long-term safety of its use.
Denosumab is not inferior to ZA in delaying the first incidence of SREs, which include pathological fractures, radiotherapy to bone, surgery to bone, or spinal cord compression. With a lower risk of kidney injury, denosumab offers a better option for cancer patients with bone metastasis to prevent the incidence of SREs. Both denosumab and ZA also demonstrate pain reduction and prevention effects in cancer patients at risk of SREs.